Electrons
Electrons are subatomic particles that orbit the nucleus of an atom. They have a negative charge and are essential to the structure of an atom. Understanding electrons is crucial to understanding the behavior of matter and the principles of electricity and magnetism.
Structure of an Atom
Atoms consist of three main subatomic particles: protons, neutrons, and electrons. Protons have a positive charge and are located in the nucleus of the atom. Neutrons have no charge and are also found in the nucleus. Electrons are much smaller than protons and neutrons and have a negative charge. They orbit the nucleus in specific energy levels or shells.
Properties of Electrons
Electrons have several important properties:
- Charge: Electrons have a negative charge, which is equal in magnitude to the positive charge of a proton. This negative charge is what allows electrons to interact with other particles and create electricity.
- Mass: Electrons have a very small mass compared to protons and neutrons. The mass of an electron is approximately 1/1836th the mass of a proton.
- Energy Levels: Electrons occupy specific energy levels or shells around the nucleus. These energy levels are designated by quantum numbers and determine the chemical properties of an element.
- Behavior: Electrons exhibit both particle-like and wave-like behavior, as described by the principles of quantum mechanics.
Role of Electrons in Chemistry and Electricity
Electrons play a crucial role in determining the chemical behavior of atoms and molecules. The arrangement of electrons in the outermost energy level of an atom determines its chemical properties and its ability to form bonds with other atoms.
In addition, the movement of electrons within a conductor is what creates electricity. When electrons flow through a material, they form an electric current, which is the basis for the functioning of electronic devices and electrical systems.
Study Guide
To understand electrons, it's important to focus on the following key areas:
- Structure of an Atom: Understand the location of electrons within an atom and how they relate to protons and neutrons.
- Properties of Electrons: Learn about the charge, mass, and energy levels of electrons, as well as their behavior as both particles and waves.
- Chemical Behavior: Explore how the arrangement of electrons in the outermost energy level influences the chemical properties of an element.
- Electricity: Investigate the role of electrons in creating electric currents and their significance in electronic devices.
◂Science Worksheets and Study Guides Fourth Grade. Cells- The building blocks of living things

 Activity Lesson
Activity Lesson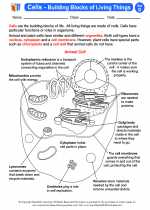
 Worksheet/Answer key
Worksheet/Answer key
 Worksheet/Answer key
Worksheet/Answer key
 Worksheet/Answer key
Worksheet/Answer key
 Worksheet/Answer key
Worksheet/Answer key
 Worksheet/Answer key
Worksheet/Answer key
 Vocabulary/Answer key
Vocabulary/Answer key
 Vocabulary/Answer key
Vocabulary/Answer key
 Vocabulary/Answer key
Vocabulary/Answer key
