Freezing Point
The freezing point of a substance is the temperature at which it changes from a liquid to a solid state at a given pressure. In other words, it is the temperature at which the solid and liquid phases of a substance are in equilibrium. For pure substances, the freezing point remains constant under a given pressure. However, for a solution, the freezing point is lower than that of the pure solvent due to the presence of solute particles.
Factors Affecting Freezing Point
Several factors can affect the freezing point of a substance:
- Pressure: Changes in pressure can affect the freezing point of a substance. Generally, an increase in pressure will lower the freezing point, while a decrease in pressure will raise the freezing point.
- Presence of impurities: When a solute is added to a solvent, the freezing point of the solution is lower than that of the pure solvent. This phenomenon is known as freezing point depression.
- Type of substance: Different substances have different freezing points. For example, water freezes at 0°C (32°F), while ethanol freezes at -114°C (-173°F).
Applications of Freezing Point
Knowledge of freezing points is essential in numerous practical applications, such as:
- Food preservation: Freezing is a common method of preserving food, as it inhibits the growth of microorganisms and slows down chemical reactions.
- Automotive industry: Antifreeze, a solution used in vehicle cooling systems, lowers the freezing point of water, preventing it from solidifying in cold temperatures.
- Chemical processes: Many industrial processes involve controlling the freezing points of substances to achieve specific physical and chemical properties.
Study Guide
Here are some key points to remember when studying the freezing point:
- Define the freezing point of a substance and understand the concept of equilibrium between solid and liquid phases.
- Understand the factors that can affect the freezing point, including pressure, presence of impurities, and the type of substance.
- Be familiar with real-life applications of freezing point in various fields, such as food preservation, automotive industry, and chemical processes.
Understanding the freezing point is crucial in many scientific and industrial contexts, and it forms the basis for numerous practical applications.
.◂Science Worksheets and Study Guides Fourth Grade. Cells- The building blocks of living things

 Activity Lesson
Activity Lesson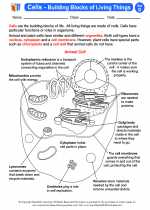
 Worksheet/Answer key
Worksheet/Answer key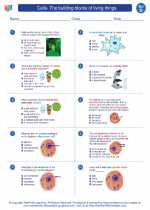
 Worksheet/Answer key
Worksheet/Answer key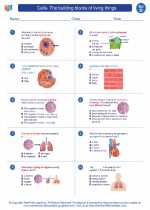
 Worksheet/Answer key
Worksheet/Answer key
 Worksheet/Answer key
Worksheet/Answer key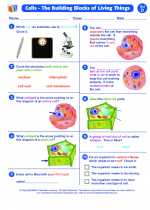
 Worksheet/Answer key
Worksheet/Answer key
 Vocabulary/Answer key
Vocabulary/Answer key
 Vocabulary/Answer key
Vocabulary/Answer key
 Vocabulary/Answer key
Vocabulary/Answer key
