P1 / T1 = P2 / T2
Where:- P1 = initial pressure of the gas
- T1 = initial temperature of the gas (in Kelvin)
- P2 = final pressure of the gas
- T2 = final temperature of the gas (in Kelvin)
2.0 atm / 300 K = P2 / 350 K
Solving for P2:P2 = (2.0 atm * 350 K) / 300 K
P2 = 2.33 atm
Therefore, the pressure of the gas at 350 K will be 2.33 atm.2. A gas at a pressure of 3.0 atm and a temperature of 400 K is cooled until its pressure is 2.0 atm. What is the final temperature of the gas, assuming the volume remains constant? Using Gay-Lussac's Law: P1 / T1 = P2 / T23.0 atm / 400 K = 2.0 atm / T2
Solving for T2:T2 = (2.0 atm * 400 K) / 3.0 atm
T2 = 266.67 K
[Gay-lussac's Law] Related Worksheets and Study Guides:
.◂Science Worksheets and Study Guides Fourth Grade. Cells- The building blocks of living things
Study Guide Cells- The building blocks of living things
Cells- The building blocks of living things  Activity Lesson
Activity Lesson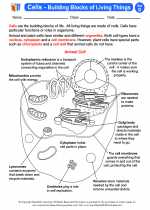 Cells - Building Blocks of Living Things
Cells - Building Blocks of Living Things  Worksheet/Answer key
Worksheet/Answer key Cells- The building blocks of living things
Cells- The building blocks of living things  Worksheet/Answer key
Worksheet/Answer key Cells- The building blocks of living things
Cells- The building blocks of living things  Worksheet/Answer key
Worksheet/Answer key Cells- The building blocks of living things
Cells- The building blocks of living things  Worksheet/Answer key
Worksheet/Answer key Cells - The Building Blocks of Living Things
Cells - The Building Blocks of Living Things  Worksheet/Answer key
Worksheet/Answer key Focus In
Focus In  Vocabulary/Answer key
Vocabulary/Answer key Cells- The building blocks of living things
Cells- The building blocks of living things  Vocabulary/Answer key
Vocabulary/Answer key Cells- The building blocks of living things
Cells- The building blocks of living things  Vocabulary/Answer key
Vocabulary/Answer key Cells- The building blocks of living things
Cells- The building blocks of living things 

 Activity Lesson
Activity Lesson
 Worksheet/Answer key
Worksheet/Answer key
 Worksheet/Answer key
Worksheet/Answer key
 Worksheet/Answer key
Worksheet/Answer key
 Worksheet/Answer key
Worksheet/Answer key
 Worksheet/Answer key
Worksheet/Answer key
 Vocabulary/Answer key
Vocabulary/Answer key
 Vocabulary/Answer key
Vocabulary/Answer key
 Vocabulary/Answer key
Vocabulary/Answer key

The resources above cover the following skills:
The Living Environment: Students understand that cells are the basic unit of life, that all life as we know it has evolved through genetic transfer and natural selection to create a great diversity of organisms, and that these organisms create interdependent webs through which matter and energy flow. Students understand similarities and differences between humans and other organisms and the interconnections of these interdependent webs.
Cells: Students describe how living things are made up of one or more cells and the ways cells help organisms meet their basic needs.
Give examples of organisms that consist of a single cell and organisms that are made of a collection of cells.