What is Glass?
Glass is a non-crystalline, amorphous solid material that is often transparent and has widespread practical, technological, and decorative uses in everyday life. It is made by melting together sand, soda ash, and limestone at high temperatures. The resulting material is then rapidly cooled to form a solid without crystallization.
Properties of Glass
Some key properties of glass include:
- Transparency: Glass allows light to pass through it, making it useful for windows, eyeglasses, and lenses.
- Brittleness: Glass is hard and rigid, but it can also break or shatter under stress.
- Chemical Inertness: Glass is resistant to most chemical reactions, making it suitable for laboratory equipment and storage containers.
- Thermal Insulation: Glass has low thermal conductivity, making it a good insulator for buildings and containers.
Types of Glass
There are several types of glass, each with unique properties and uses:
- Soda-Lime Glass: The most common type of glass used in windows, bottles, and glassware.
- Borosilicate Glass: Known for its high resistance to thermal shock, used in laboratory glassware and cookware.
- Tempered Glass: Treated for increased strength and safety, often used in car windows and shower doors.
- Float Glass: Produced by floating molten glass on a bed of molten metal, used for architectural purposes.
Uses of Glass
Glass has a wide range of applications, including:
- Construction: Windows, doors, and architectural features.
- Household Items: Drinkware, cookware, and decorative ornaments.
- Technology: Screens, lenses, and optical fibers.
- Industry: Laboratory equipment, containers, and insulation.
Study Guide
When studying the topic of glass, it's important to understand its composition, properties, types, and uses. Here are some key points to focus on:
- What are the main components used to make glass?
- Describe the properties of glass and give examples of how these properties are utilized in everyday life.
- Identify and explain the different types of glass, along with their specific applications.
- Discuss the various uses of glass across different industries and settings.
◂Science Worksheets and Study Guides Fourth Grade. Electricity and magnetism
Study Guide Electricity and magnetism
Electricity and magnetism  Activity Lesson
Activity Lesson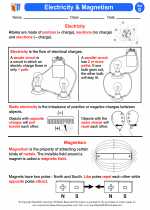 Electricity & Magnetism
Electricity & Magnetism  Worksheet/Answer key
Worksheet/Answer key Electricity and magnetism
Electricity and magnetism  Worksheet/Answer key
Worksheet/Answer key Electricity and magnetism
Electricity and magnetism  Worksheet/Answer key
Worksheet/Answer key Electricity and magnetism
Electricity and magnetism  Worksheet/Answer key
Worksheet/Answer key Electricity and Magnetism
Electricity and Magnetism  Vocabulary/Answer key
Vocabulary/Answer key Electricity and magnetism
Electricity and magnetism  Vocabulary/Answer key
Vocabulary/Answer key Electricity and magnetism
Electricity and magnetism 

 Activity Lesson
Activity Lesson
 Worksheet/Answer key
Worksheet/Answer key
 Worksheet/Answer key
Worksheet/Answer key
 Worksheet/Answer key
Worksheet/Answer key
 Worksheet/Answer key
Worksheet/Answer key
 Vocabulary/Answer key
Vocabulary/Answer key
 Vocabulary/Answer key
Vocabulary/Answer key

The resources above cover the following skills:
PHYSICAL SCIENCE
Energy
Plan and carry out investigations that explain transference of energy from place to place by sound, light, heat, and electric currents.
Demonstrate that electric circuits require a complete loop through which an electric current can pass.
Design, construct, and test a device that changes energy from one form to another (e.g., electric circuits converting electrical energy into motion, light, or sound energy; a passive solar heater converting light energy into heat energy).