High Specific Heat
Specific heat is the amount of heat required to raise the temperature of a substance by a certain amount. A high specific heat means that a substance requires a large amount of heat to increase its temperature.
Explanation:
Substances with high specific heat can absorb and release a large amount of heat energy with minimal temperature change. Water, for example, has a high specific heat which allows it to moderate the temperature of the environment, making it an important factor in climate regulation.
Study Guide:
- Definition: Specific heat is the amount of heat required to raise the temperature of a substance by a certain amount.
- High Specific Heat: A high specific heat means that a substance requires a large amount of heat to increase its temperature. This property allows the substance to absorb and release a large amount of heat energy with minimal temperature change.
- Importance of High Specific Heat: High specific heat is important in regulating temperature in natural systems, such as bodies of water and the Earth's atmosphere.
- Examples: Water is a commonly known substance with a high specific heat, which allows it to retain heat and moderate temperature changes in the environment.
- Application: Understanding the concept of high specific heat is important in fields such as climate science, engineering, and environmental studies.
◂Science Worksheets and Study Guides Fourth Grade. Organ systems
Study Guide Organ systems
Organ systems  Worksheet/Answer key
Worksheet/Answer key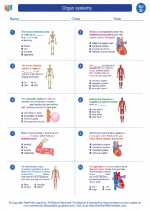 Organ systems
Organ systems  Worksheet/Answer key
Worksheet/Answer key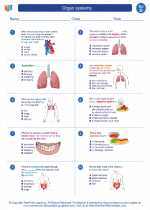 Organ systems
Organ systems  Worksheet/Answer key
Worksheet/Answer key Organ systems
Organ systems  Vocabulary/Answer key
Vocabulary/Answer key Organ systems
Organ systems  Vocabulary/Answer key
Vocabulary/Answer key Organ systems
Organ systems  Vocabulary/Answer key
Vocabulary/Answer key Organ systems
Organ systems  Vocabulary/Answer key
Vocabulary/Answer key Organ systems
Organ systems  Vocabulary/Answer key
Vocabulary/Answer key Organ systems
Organ systems 

 Worksheet/Answer key
Worksheet/Answer key
 Worksheet/Answer key
Worksheet/Answer key
 Worksheet/Answer key
Worksheet/Answer key
 Vocabulary/Answer key
Vocabulary/Answer key
 Vocabulary/Answer key
Vocabulary/Answer key
 Vocabulary/Answer key
Vocabulary/Answer key
 Vocabulary/Answer key
Vocabulary/Answer key
 Vocabulary/Answer key
Vocabulary/Answer key

The resources above cover the following skills:
LIFE SCIENCE (NGSS)
From Molecules to Organisms: Structures and Processes
Students who demonstrate understanding can:
Construct an argument that plants and animals have internal and external structures that function to support survival, growth, behavior, and reproduction.