Endothermic Reactions
An endothermic reaction is a chemical reaction that absorbs heat from its surroundings. This means that the surroundings become cooler as the reaction occurs, as the energy is being taken in rather than released. Endothermic reactions require an input of energy to proceed, and they are often associated with a decrease in temperature.
Examples of Endothermic Reactions:
Factors Affecting Endothermic Reactions:
Several factors can affect the rate and extent of endothermic reactions, including:
- Temperature: Higher temperatures can increase the rate of endothermic reactions, as they provide more energy for the reaction to absorb.
- Concentration of reactants: Increasing the concentration of reactants can lead to a faster reaction, as there are more particles available to react.
- Catalysts: Certain substances can speed up endothermic reactions without being consumed in the process.
Study Guide:
When studying endothermic reactions, it's important to understand the following key points:
- Define endothermic reactions and explain the concept of heat absorption.
- Identify examples of endothermic reactions in everyday life and in chemical processes.
- Understand the factors that can affect the rate and extent of endothermic reactions.
- Be able to differentiate between endothermic and exothermic reactions, and identify their respective characteristics.
As you study, make sure to practice identifying endothermic reactions and understanding the role of energy in chemical processes. You can also conduct experiments to observe and measure the temperature changes associated with different endothermic reactions.
Remember to review the key concepts and examples to solidify your understanding of endothermic reactions.
.◂Science Worksheets and Study Guides Fifth Grade. Cells, tissues and organs

 Worksheet/Answer key
Worksheet/Answer key
 Worksheet/Answer key
Worksheet/Answer key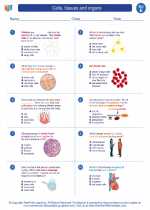
 Worksheet/Answer key
Worksheet/Answer key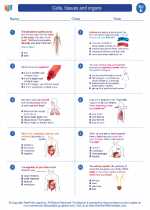
 Vocabulary/Answer key
Vocabulary/Answer key
 Vocabulary/Answer key
Vocabulary/Answer key
 Vocabulary/Answer key
Vocabulary/Answer key
 Vocabulary/Answer key
Vocabulary/Answer key
 Vocabulary/Answer key
Vocabulary/Answer key
 Vocabulary/Answer key
Vocabulary/Answer key
