Bases
In chemistry, bases are substances that have a pH greater than 7. They are the opposite of acids, which have a pH lower than 7. Bases are also known as alkaline substances. They have a bitter taste and are slippery to the touch. Some common examples of bases include sodium hydroxide (NaOH), potassium hydroxide (KOH), and ammonia (NH3).
Properties of Bases
- Bases turn red litmus paper blue.
- They are proton acceptors, meaning they can accept hydrogen ions (H+) in a chemical reaction.
- Many bases are also good conductors of electricity when dissolved in water, as they produce hydroxide ions (OH-) which allow the flow of electric current.
- They have a soapy or slippery feel.
Common Bases
| Base | Chemical Formula |
|---|---|
| Sodium hydroxide | NaOH |
| Potassium hydroxide | KOH |
| Ammonia | NH3 |
Uses of Bases
Bases have various industrial and household uses. Some common applications include:
- Manufacture of soaps and detergents
- Neutralizing acidic substances
- Cleaning and disinfecting
- Controlling pH in agricultural practices
Study Guide
To study bases, it's important to understand their properties, common examples, and uses. Here are some key points to focus on:
- Define bases and their characteristics.
- Learn to identify bases using indicators such as litmus paper.
- Understand the concept of pH and how bases contribute to basic solutions.
- Memorize common examples of bases and their chemical formulas.
- Explore the various applications of bases in everyday life and industries.
[Bases] Related Worksheets and Study Guides:
.◂Science Worksheets and Study Guides Fifth Grade. Flowers and seeds
Study Guide Flowers and seeds
Flowers and seeds  Activity Lesson
Activity Lesson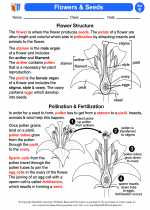 Flowers & Seeds
Flowers & Seeds  Worksheet/Answer key
Worksheet/Answer key Flowers and seeds
Flowers and seeds  Worksheet/Answer key
Worksheet/Answer key Flowers and seeds
Flowers and seeds  Worksheet/Answer key
Worksheet/Answer key Flowers and seeds
Flowers and seeds  Worksheet/Answer key
Worksheet/Answer key Flowers and seeds
Flowers and seeds  Vocabulary/Answer key
Vocabulary/Answer key Flowers and seeds
Flowers and seeds  Vocabulary/Answer key
Vocabulary/Answer key Flowers and seeds
Flowers and seeds 

 Activity Lesson
Activity Lesson
 Worksheet/Answer key
Worksheet/Answer key
 Worksheet/Answer key
Worksheet/Answer key
 Worksheet/Answer key
Worksheet/Answer key
 Worksheet/Answer key
Worksheet/Answer key
 Vocabulary/Answer key
Vocabulary/Answer key
 Vocabulary/Answer key
Vocabulary/Answer key

The resources above cover the following skills:
Life Science
All organisms have structures and systems with separate functions. Students can:
Develop and communicate an evidence-based scientific explanation of the role of different organs or structures that are important for an organism's survival - in both plants and animals