Flowers and seeds -> combustion reactions
Combustion Reactions
Combustion is a chemical reaction that occurs between a fuel and an oxidizing agent, usually oxygen. The reaction releases heat and light in the form of a flame. This type of reaction is exothermic, meaning it releases energy in the form of heat.
Key Points to Remember:
- Combustion reactions require a fuel and an oxidizing agent, usually oxygen.
- Common fuels involved in combustion reactions include hydrocarbons such as methane, propane, and gasoline.
- The products of a combustion reaction are carbon dioxide and water, along with heat and light.
- Combustion reactions are essential for processes such as burning wood for heat, fueling engines, and providing energy for various industrial processes.
Examples of Combustion Reactions:
Here are some common examples of combustion reactions:
- Burning of natural gas: CH4 + 2O2 → CO2 + 2H2O
- Burning of propane: C3H8 + 5O2 → 3CO2 + 4H2O
- Burning of gasoline: C8H18 + 25O2 → 8CO2 + 9H2O
Study Guide:
Here are some key points to include in your study of combustion reactions:
- Understand the definition of combustion and the key components involved in the reaction.
- Learn to identify common fuels and oxidizing agents used in combustion reactions.
- Practice balancing combustion equations to understand the stoichiometry of the reaction.
- Explore real-world applications of combustion reactions, such as in heating systems, engines, and industrial processes.
- Understand the environmental impact of combustion reactions, particularly in terms of air pollution and greenhouse gas emissions.
Remember to review these key points and practice solving combustion reaction problems to reinforce your understanding of the topic.
[Combustion Reactions] Related Worksheets and Study Guides:
.◂Science Worksheets and Study Guides Fifth Grade. Flowers and seeds
Study Guide Flowers and seeds
Flowers and seeds  Activity Lesson
Activity Lesson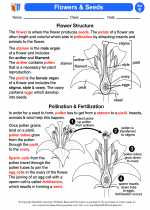 Flowers & Seeds
Flowers & Seeds  Worksheet/Answer key
Worksheet/Answer key Flowers and seeds
Flowers and seeds  Worksheet/Answer key
Worksheet/Answer key Flowers and seeds
Flowers and seeds  Worksheet/Answer key
Worksheet/Answer key Flowers and seeds
Flowers and seeds  Worksheet/Answer key
Worksheet/Answer key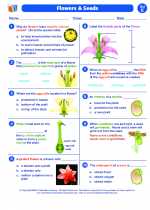 Flowers and seeds
Flowers and seeds  Vocabulary/Answer key
Vocabulary/Answer key Flowers and seeds
Flowers and seeds  Vocabulary/Answer key
Vocabulary/Answer key Flowers and seeds
Flowers and seeds 

 Activity Lesson
Activity Lesson
 Worksheet/Answer key
Worksheet/Answer key
 Worksheet/Answer key
Worksheet/Answer key
 Worksheet/Answer key
Worksheet/Answer key
 Worksheet/Answer key
Worksheet/Answer key
 Vocabulary/Answer key
Vocabulary/Answer key
 Vocabulary/Answer key
Vocabulary/Answer key

The resources above cover the following skills:
Life Science
All organisms have structures and systems with separate functions. Students can:
Develop and communicate an evidence-based scientific explanation of the role of different organs or structures that are important for an organism's survival - in both plants and animals