Density
Density is a measure of how much mass is contained in a given volume. It is calculated by dividing the mass of an object by its volume. The formula for density is:
center;">Density = Mass / Volume
Density is a fundamental property of matter and can be used to identify and compare different substances. It is typically measured in units such as grams per cubic centimeter (g/cm3) for solids and liquids, and kilograms per cubic meter (kg/m3) for gases.
Factors Affecting Density
The density of a substance can be affected by changes in temperature and pressure. Generally, as the temperature of a substance increases, its density decreases, and as the pressure increases, the density also increases.
Examples of Density
Some common examples of density include:
- Ice has a lower density than water, which is why it floats.
- Iron has a higher density than wood, which is why iron sinks while wood floats.
- Helium gas has a lower density than air, which is why helium-filled balloons float in air.
Applications of Density
The concept of density has wide-ranging applications in various fields, such as:
- Identifying unknown substances through density measurements.
- Determining the purity of a substance by comparing its measured density to the known density of the pure material.
- Engineering and construction, where knowledge of the density of materials is crucial for designing structures and selecting appropriate building materials.
Study Guide
To understand and master the concept of density, here are some key points to focus on:
- Understand the definition of density and how it is calculated.
- Learn the units of measurement for density and how to convert between different units.
- Explore real-life examples and applications of density to appreciate its significance.
- Practice solving problems involving density, mass, and volume to reinforce understanding.
- Experiment with different materials to observe and compare their densities.
By grasping these fundamental aspects of density, you will develop a strong foundation in this important concept of physical science.
[Density] Related Worksheets and Study Guides:
.◂Science Worksheets and Study Guides Fifth Grade. Flowers and seeds

 Activity Lesson
Activity Lesson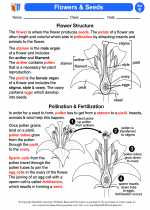
 Worksheet/Answer key
Worksheet/Answer key
 Worksheet/Answer key
Worksheet/Answer key
 Worksheet/Answer key
Worksheet/Answer key
 Worksheet/Answer key
Worksheet/Answer key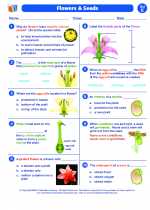
 Vocabulary/Answer key
Vocabulary/Answer key
 Vocabulary/Answer key
Vocabulary/Answer key
