Freezing
Freezing is the process in which a liquid changes into a solid as a result of a decrease in temperature. When a substance is cooled down to its freezing point, the molecules within the liquid lose energy and slow down, causing them to arrange themselves into a more ordered and stable structure, resulting in the formation of a solid.
Key Concepts:
- Freezing Point: The temperature at which a substance changes from a liquid to a solid. It is a characteristic property of the substance.
- Crystal Formation: During freezing, the molecules of the liquid arrange themselves into a specific crystal lattice structure, resulting in the formation of a solid with a specific geometric shape.
- Latent Heat of Fusion: The amount of heat energy required to convert a unit mass of a substance from a solid to a liquid at its melting point, or vice versa.
Study Guide:
- What is freezing and how does it occur?
- What factors can affect the freezing point of a substance?
- Compare and contrast the molecular arrangement of a liquid and a solid during freezing.
- How does the latent heat of fusion play a role in the process of freezing?
- Provide examples of substances that freeze at different temperatures.
Understanding the process of freezing is important in various scientific fields, including chemistry, physics, and environmental science. It has practical applications in areas such as food preservation, material science, and climate studies.
[Freezing] Related Worksheets and Study Guides:
.◂Science Worksheets and Study Guides Fifth Grade. Flowers and seeds
Study Guide Flowers and seeds
Flowers and seeds  Activity Lesson
Activity Lesson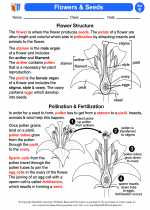 Flowers & Seeds
Flowers & Seeds  Worksheet/Answer key
Worksheet/Answer key Flowers and seeds
Flowers and seeds  Worksheet/Answer key
Worksheet/Answer key Flowers and seeds
Flowers and seeds  Worksheet/Answer key
Worksheet/Answer key Flowers and seeds
Flowers and seeds  Worksheet/Answer key
Worksheet/Answer key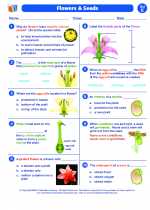 Flowers and seeds
Flowers and seeds  Vocabulary/Answer key
Vocabulary/Answer key Flowers and seeds
Flowers and seeds  Vocabulary/Answer key
Vocabulary/Answer key Flowers and seeds
Flowers and seeds 

 Activity Lesson
Activity Lesson
 Worksheet/Answer key
Worksheet/Answer key
 Worksheet/Answer key
Worksheet/Answer key
 Worksheet/Answer key
Worksheet/Answer key
 Worksheet/Answer key
Worksheet/Answer key
 Vocabulary/Answer key
Vocabulary/Answer key
 Vocabulary/Answer key
Vocabulary/Answer key

The resources above cover the following skills:
Life Science
All organisms have structures and systems with separate functions. Students can:
Develop and communicate an evidence-based scientific explanation of the role of different organs or structures that are important for an organism's survival - in both plants and animals