Gas
Gas is one of the four fundamental states of matter. It is composed of molecules that are in constant, random motion and are able to spread out to fill the space available to them. Here are some key points to understand about gases:
- Gas particles: Gas particles are in constant motion and move in straight lines until they collide with another particle or the walls of their container.
- Volume and shape: Gases have neither a definite volume nor a definite shape. They expand to fill the entire volume of their container and take its shape.
- Pressure: The pressure of a gas is the force exerted by the gas on the walls of its container. It is caused by the constant motion and collisions of the gas particles.
- Temperature and gas behavior: Increasing the temperature of a gas will increase the average speed of its particles, causing the gas to expand. Decreasing the temperature will have the opposite effect, causing the gas to contract.
- Gas laws: There are several gas laws that describe the behavior of gases under different conditions, including Boyle's Law, Charles's Law, and the Ideal Gas Law.
Study Guide:
Here are some key concepts and questions to consider when studying gases:
- Describe the behavior of gas particles at the molecular level.
- Explain the relationship between temperature and the behavior of gases.
- Discuss the factors that affect the pressure of a gas.
- Summarize the main principles of Boyle's Law and how it relates to the behavior of gases.
- Compare and contrast the behavior of gases with that of solids and liquids.
Understanding the behavior of gases is important in fields such as chemistry, physics, and engineering. By grasping the fundamental principles of gases, you can gain insights into various natural phenomena and practical applications.
[Gas] Related Worksheets and Study Guides:
.◂Science Worksheets and Study Guides Fifth Grade. Flowers and seeds
Study Guide Flowers and seeds
Flowers and seeds  Activity Lesson
Activity Lesson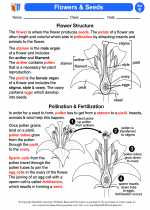 Flowers & Seeds
Flowers & Seeds  Worksheet/Answer key
Worksheet/Answer key Flowers and seeds
Flowers and seeds  Worksheet/Answer key
Worksheet/Answer key Flowers and seeds
Flowers and seeds  Worksheet/Answer key
Worksheet/Answer key Flowers and seeds
Flowers and seeds  Worksheet/Answer key
Worksheet/Answer key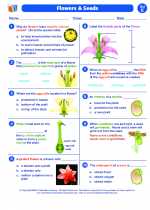 Flowers and seeds
Flowers and seeds  Vocabulary/Answer key
Vocabulary/Answer key Flowers and seeds
Flowers and seeds  Vocabulary/Answer key
Vocabulary/Answer key Flowers and seeds
Flowers and seeds 

 Activity Lesson
Activity Lesson
 Worksheet/Answer key
Worksheet/Answer key
 Worksheet/Answer key
Worksheet/Answer key
 Worksheet/Answer key
Worksheet/Answer key
 Worksheet/Answer key
Worksheet/Answer key
 Vocabulary/Answer key
Vocabulary/Answer key
 Vocabulary/Answer key
Vocabulary/Answer key

The resources above cover the following skills:
Life Science
All organisms have structures and systems with separate functions. Students can:
Develop and communicate an evidence-based scientific explanation of the role of different organs or structures that are important for an organism's survival - in both plants and animals