Ice
Ice is the solid form of water that forms when water freezes at a temperature of 0 degrees Celsius (32 degrees Fahrenheit). It is found in glaciers, icebergs, and frozen bodies of water such as lakes and rivers. Ice is also commonly used for cooling drinks and preserving food.
Properties of Ice
- Physical State: Ice is a solid at temperatures below 0 degrees Celsius.
- Crystal Structure: Ice has a hexagonal crystal structure, which gives it its unique pattern of six-sided shapes.
- Density: Ice is less dense than liquid water, which is why it floats on water.
- Transparency: Ice is transparent, allowing light to pass through it.
Formation of Ice
Ice forms when water molecules slow down and come together to form a crystalline structure. This occurs when the temperature drops below the freezing point of water, causing the molecules to arrange themselves into a solid lattice.
Uses of Ice
Ice has many practical uses, including:
- Preserving and storing food
- Cooling drinks
- Creating artificial skating rinks
- Ice sculpting and carving
- Scientific research and experiments
Study Guide Questions
- What is the temperature at which water freezes to form ice?
- Describe the crystal structure of ice.
- Why does ice float on water?
- What are some practical uses of ice?
- Explain the process of ice formation.
These study guide questions will help you reinforce your understanding of the properties and uses of ice.
[Ice] Related Worksheets and Study Guides:
.◂Science Worksheets and Study Guides Fifth Grade. Flowers and seeds
Study Guide Flowers and seeds
Flowers and seeds  Activity Lesson
Activity Lesson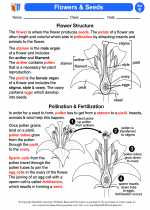 Flowers & Seeds
Flowers & Seeds  Worksheet/Answer key
Worksheet/Answer key Flowers and seeds
Flowers and seeds  Worksheet/Answer key
Worksheet/Answer key Flowers and seeds
Flowers and seeds  Worksheet/Answer key
Worksheet/Answer key Flowers and seeds
Flowers and seeds  Worksheet/Answer key
Worksheet/Answer key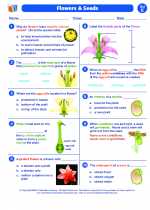 Flowers and seeds
Flowers and seeds  Vocabulary/Answer key
Vocabulary/Answer key Flowers and seeds
Flowers and seeds  Vocabulary/Answer key
Vocabulary/Answer key Flowers and seeds
Flowers and seeds 

 Activity Lesson
Activity Lesson
 Worksheet/Answer key
Worksheet/Answer key
 Worksheet/Answer key
Worksheet/Answer key
 Worksheet/Answer key
Worksheet/Answer key
 Worksheet/Answer key
Worksheet/Answer key
 Vocabulary/Answer key
Vocabulary/Answer key
 Vocabulary/Answer key
Vocabulary/Answer key

The resources above cover the following skills:
Life Science
All organisms have structures and systems with separate functions. Students can:
Develop and communicate an evidence-based scientific explanation of the role of different organs or structures that are important for an organism's survival - in both plants and animals