Isotopes
Isotopes are atoms of the same element that have the same number of protons but different numbers of neutrons. This means that isotopes of an element have the same atomic number but different mass numbers. For example, hydrogen has three isotopes: protium, deuterium, and tritium.
Key Points to Remember:
- Isotopes have the same number of protons but different numbers of neutrons.
- Isotopes of an element have the same atomic number but different mass numbers.
- Isotopes can be stable or radioactive.
- Isotopes are used in various fields such as medicine, industry, and research.
Study Guide:
Here are some key concepts to focus on when studying isotopes:
- Atomic Structure: Understand the basic structure of an atom, including the arrangement of protons, neutrons, and electrons.
- Isotopes and Mass Number: Learn how to determine the number of protons, neutrons, and electrons in an isotope based on its mass number.
- Stability and Radioactivity: Explore the concept of stable isotopes versus radioactive isotopes, and the implications of radioactivity in various applications.
- Applications of Isotopes: Research the different ways in which isotopes are used in fields such as medicine (e.g., medical imaging, cancer treatment), industry (e.g., agriculture, environmental monitoring), and research (e.g., carbon dating).
- Isotopic Notation: Practice writing isotopic notation for different isotopes, including the element symbol, atomic number, and mass number.
By understanding these key points and concepts, you will develop a strong foundation in the topic of isotopes and their significance in the study of chemistry and beyond.
[Isotopes] Related Worksheets and Study Guides:
.◂Science Worksheets and Study Guides Fifth Grade. Flowers and seeds
Study Guide Flowers and seeds
Flowers and seeds  Activity Lesson
Activity Lesson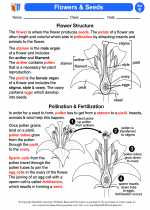 Flowers & Seeds
Flowers & Seeds  Worksheet/Answer key
Worksheet/Answer key Flowers and seeds
Flowers and seeds  Worksheet/Answer key
Worksheet/Answer key Flowers and seeds
Flowers and seeds  Worksheet/Answer key
Worksheet/Answer key Flowers and seeds
Flowers and seeds  Worksheet/Answer key
Worksheet/Answer key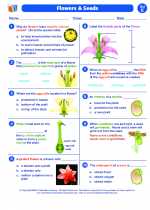 Flowers and seeds
Flowers and seeds  Vocabulary/Answer key
Vocabulary/Answer key Flowers and seeds
Flowers and seeds  Vocabulary/Answer key
Vocabulary/Answer key Flowers and seeds
Flowers and seeds 

 Activity Lesson
Activity Lesson
 Worksheet/Answer key
Worksheet/Answer key
 Worksheet/Answer key
Worksheet/Answer key
 Worksheet/Answer key
Worksheet/Answer key
 Worksheet/Answer key
Worksheet/Answer key
 Vocabulary/Answer key
Vocabulary/Answer key
 Vocabulary/Answer key
Vocabulary/Answer key

The resources above cover the following skills:
Life Science
All organisms have structures and systems with separate functions. Students can:
Develop and communicate an evidence-based scientific explanation of the role of different organs or structures that are important for an organism's survival - in both plants and animals