Sodium
Sodium is a chemical element with the symbol Na and atomic number 11. It is a soft, silvery-white, highly reactive metal and is a member of the alkali metals. Sodium is essential for human health and is found in many foods. It plays a crucial role in the functioning of nerves and muscles.
Physical Properties of Sodium
- Symbol: Na
- Atomic number: 11
- Atomic mass: 22.989769 u
- State at room temperature: Solid
- Melting point: 97.79°C (208.02°F)
- Boiling point: 882.9°C (1621.2°F)
Chemical Properties of Sodium
Sodium is highly reactive and reacts vigorously with water, producing hydrogen gas and heat. It also reacts with oxygen to form sodium oxide. In its pure form, sodium is stored under oil to prevent it from reacting with moisture in the air.
Uses of Sodium
Sodium has numerous uses, including:
- Manufacturing of chemicals such as sodium hydroxide and sodium carbonate
- Production of metal alloys
- As a coolant in nuclear reactors
- As a component in some pharmaceuticals and food products
- Sodium is a soft, silvery-white, highly reactive metal.
- It is a member of the alkali metals and has the symbol Na and atomic number 11.
- Sodium is essential for human health and is found in many foods.
- It plays a crucial role in the functioning of nerves and muscles.
- Sodium reacts vigorously with water and oxygen, and is stored under oil to prevent reactions with moisture in the air.
- It is used in the manufacturing of chemicals, production of metal alloys, as a coolant in nuclear reactors, and in some pharmaceuticals and food products.
Study Guide
Here are some key points to remember about sodium:
Remember to handle sodium with care due to its reactivity, and be mindful of its importance in maintaining a healthy balance in the human body.
.◂Science Worksheets and Study Guides Fifth Grade. Flowers and seeds

 Activity Lesson
Activity Lesson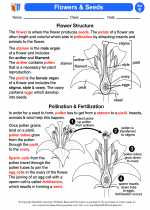
 Worksheet/Answer key
Worksheet/Answer key
 Worksheet/Answer key
Worksheet/Answer key
 Worksheet/Answer key
Worksheet/Answer key
 Worksheet/Answer key
Worksheet/Answer key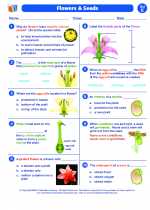
 Vocabulary/Answer key
Vocabulary/Answer key
 Vocabulary/Answer key
Vocabulary/Answer key
