Solid
A solid is one of the three main states of matter, along with liquid and gas. Solids have a definite shape and volume. The particles in a solid are tightly packed together and vibrate in place. The arrangement and movement of these particles give solids their specific properties and characteristics.
Characteristics of Solids:
- Definite Shape: Solids have a fixed, definite shape that does not change unless acted upon by an external force.
- Definite Volume: The volume of a solid remains constant, as the particles are closely packed together.
- Resistance to Flow: Unlike liquids and gases, solids do not flow and maintain their shape under normal conditions.
- Strong Intermolecular Forces: The particles in a solid are held together by strong intermolecular forces, contributing to the solid's rigidity.
- Crystalline or Amorphous: Solids can be classified as crystalline (having a highly ordered arrangement of particles) or amorphous (lacking a regular, ordered structure).
Examples of Solids:
Common examples of solids include ice, wood, metal, plastic, and rock. These substances maintain their shape and volume under normal conditions, exhibiting the characteristic properties of solids.
Study Guide:
Here are some key points to remember when studying solids:
- Define what a solid is and its key characteristics.
- Understand the difference between crystalline and amorphous solids.
- Learn about the arrangement and movement of particles in a solid.
- Explore real-life examples of solids and how they exhibit the properties of this state of matter.
- Consider the applications and uses of solids in everyday life and various industries.
[Solid] Related Worksheets and Study Guides:
.◂Science Worksheets and Study Guides Fifth Grade. Flowers and seeds
Study Guide Flowers and seeds
Flowers and seeds  Activity Lesson
Activity Lesson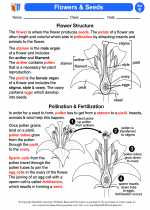 Flowers & Seeds
Flowers & Seeds  Worksheet/Answer key
Worksheet/Answer key Flowers and seeds
Flowers and seeds  Worksheet/Answer key
Worksheet/Answer key Flowers and seeds
Flowers and seeds  Worksheet/Answer key
Worksheet/Answer key Flowers and seeds
Flowers and seeds  Worksheet/Answer key
Worksheet/Answer key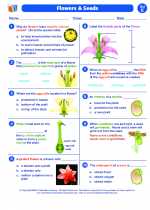 Flowers and seeds
Flowers and seeds  Vocabulary/Answer key
Vocabulary/Answer key Flowers and seeds
Flowers and seeds  Vocabulary/Answer key
Vocabulary/Answer key Flowers and seeds
Flowers and seeds 

 Activity Lesson
Activity Lesson
 Worksheet/Answer key
Worksheet/Answer key
 Worksheet/Answer key
Worksheet/Answer key
 Worksheet/Answer key
Worksheet/Answer key
 Worksheet/Answer key
Worksheet/Answer key
 Vocabulary/Answer key
Vocabulary/Answer key
 Vocabulary/Answer key
Vocabulary/Answer key

The resources above cover the following skills:
Life Science
All organisms have structures and systems with separate functions. Students can:
Develop and communicate an evidence-based scientific explanation of the role of different organs or structures that are important for an organism's survival - in both plants and animals