Potassium
Potassium is a chemical element with the symbol K and atomic number 19. It is a soft, silvery-white metal that reacts explosively with water and rapidly with air. Potassium is essential for all living organisms, as it plays a crucial role in various physiological processes.
Properties of Potassium:
- Physical Properties: Potassium is a soft, silver-white metal with a low melting point. It is highly reactive and is never found free in nature.
- Chemical Properties: Potassium readily reacts with water to produce hydrogen gas and potassium hydroxide. It also reacts with oxygen to form potassium oxide.
- Biological Properties: Potassium ions are vital for the functioning of all living cells. They play a key role in nerve transmission, muscle contraction, and maintaining fluid balance within the body.
Uses of Potassium:
- Health and Medicine: Potassium supplements are used to treat or prevent low potassium levels in the blood.
- Agriculture: Potassium compounds are widely used as fertilizers to improve the growth and quality of crops.
- Industrial Applications: Potassium compounds are used in the production of soaps, glass, and ceramics.
Study Guide:
- What is the atomic number of potassium?
- Describe the physical properties of potassium.
- Explain the biological importance of potassium.
- What are the main uses of potassium?
◂Science Worksheets and Study Guides Sixth Grade. Introduction to earth science
Study Guide Introduction to earth science
Introduction to earth science  Activity Lesson
Activity Lesson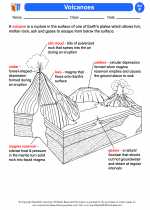 Volcanoes
Volcanoes  Worksheet/Answer key
Worksheet/Answer key Introduction to earth science
Introduction to earth science  Worksheet/Answer key
Worksheet/Answer key Introduction to earth science
Introduction to earth science  Worksheet/Answer key
Worksheet/Answer key Introduction to earth science
Introduction to earth science  Vocabulary/Answer key
Vocabulary/Answer key Introduction to earth science
Introduction to earth science  Vocabulary/Answer key
Vocabulary/Answer key Introduction to earth science
Introduction to earth science 

 Activity Lesson
Activity Lesson
 Worksheet/Answer key
Worksheet/Answer key
 Worksheet/Answer key
Worksheet/Answer key
 Worksheet/Answer key
Worksheet/Answer key
 Vocabulary/Answer key
Vocabulary/Answer key
 Vocabulary/Answer key
Vocabulary/Answer key

The resources above cover the following skills:
EARTH AND SPACE SCIENCE