Aluminum
Aluminum is a chemical element with the symbol Al and atomic number 13. It is a silvery-white, soft, nonmagnetic and ductile metal in the boron group. By mass, aluminum makes up about 8% of the Earth's crust, making it the third most abundant element after oxygen and silicon.
Properties of Aluminum
- Physical Properties: Aluminum is a lightweight metal with a density about one-third that of steel or copper. It has a low melting point and is malleable, meaning it can be easily shaped or formed.
- Chemical Properties: Aluminum is a reactive metal and is highly resistant to corrosion due to the formation of a thin layer of aluminum oxide on the surface, which acts as a protective barrier against further oxidation.
Uses of Aluminum
Aluminum is widely used in various industries due to its desirable properties:
- Construction: Aluminum is used in the construction of buildings, vehicles, and aircraft due to its lightweight and corrosion-resistant nature.
- Packaging: Aluminum foil and cans are commonly used for packaging food and beverages due to its ability to block light, oxygen, and moisture.
- Electrical Transmission: Aluminum is used in power lines and electrical wiring due to its conductivity and low cost.
- Manufacturing: It is used in the manufacturing of a wide range of products, including car parts, appliances, and machinery.
Environmental Impact
While aluminum is abundant and versatile, its production can have environmental impacts, including energy consumption and greenhouse gas emissions. Recycling aluminum can significantly reduce these impacts, as it requires only 5% of the energy used to produce new aluminum from raw materials.
Study Guide for Aluminum
- What is the atomic number of aluminum?
- What is the chemical symbol for aluminum?
- What is the most abundant element in the Earth's crust?
- List three physical properties of aluminum.
- Explain the role of aluminum oxide in preventing corrosion.
- Name three common uses of aluminum.
- How does recycling aluminum contribute to environmental sustainability?
◂Science Worksheets and Study Guides Seventh Grade. Cell Reproduction
Study Guide Cell Reproduction
Cell Reproduction  Activity Lesson
Activity Lesson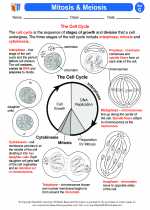 Mitosis & Meiosis
Mitosis & Meiosis  Worksheet/Answer key
Worksheet/Answer key Cell Reproduction
Cell Reproduction  Worksheet/Answer key
Worksheet/Answer key Cell Reproduction
Cell Reproduction  Worksheet/Answer key
Worksheet/Answer key Cell Reproduction
Cell Reproduction  Vocabulary/Answer key
Vocabulary/Answer key Cell Reproduction
Cell Reproduction  Vocabulary/Answer key
Vocabulary/Answer key Cell Reproduction
Cell Reproduction  Vocabulary/Answer key
Vocabulary/Answer key Cell Reproduction
Cell Reproduction  Vocabulary/Answer key
Vocabulary/Answer key Cell Reproduction
Cell Reproduction  Vocabulary/Answer key
Vocabulary/Answer key Cell Reproduction
Cell Reproduction  Vocabulary/Answer key
Vocabulary/Answer key Cell Reproduction
Cell Reproduction  Vocabulary/Answer key
Vocabulary/Answer key Cell Reproduction
Cell Reproduction  Vocabulary/Answer key
Vocabulary/Answer key Cell Reproduction
Cell Reproduction 

 Activity Lesson
Activity Lesson
 Worksheet/Answer key
Worksheet/Answer key
 Worksheet/Answer key
Worksheet/Answer key
 Worksheet/Answer key
Worksheet/Answer key
 Vocabulary/Answer key
Vocabulary/Answer key
 Vocabulary/Answer key
Vocabulary/Answer key
 Vocabulary/Answer key
Vocabulary/Answer key
 Vocabulary/Answer key
Vocabulary/Answer key
 Vocabulary/Answer key
Vocabulary/Answer key
 Vocabulary/Answer key
Vocabulary/Answer key
 Vocabulary/Answer key
Vocabulary/Answer key
 Vocabulary/Answer key
Vocabulary/Answer key

The resources above cover the following skills:
LIFE SCIENCE
From Molecules to Organisms: Structures and Processes
Gather and synthesize information to explain how prokaryotic and eukaryotic cells differ in structure and function, including the methods of asexual and sexual reproduction.