Colloids
Colloids are mixtures that contain particles that are intermediate in size between those in solutions and suspensions. These particles are larger than the particles in a solution but smaller than those in a suspension.
Types of Colloids
- Sol: A colloid in which solid particles are dispersed in a liquid.
- Gel: A colloid in which a liquid is dispersed in a solid.
- Emulsion: A colloid in which liquid droplets are dispersed in a liquid.
- Aerosol: A colloid in which liquid or solid particles are dispersed in a gas.
- Foam: A colloid in which gas is dispersed in a liquid.
Properties of Colloids
- Particle Size: The particles in a colloid are larger than molecules in a solution but smaller than those in a suspension.
- Scattering of Light: Colloidal particles scatter light, making the dispersion appear cloudy or opaque.
- Brownian Motion: Colloidal particles exhibit Brownian motion, which is the random movement of particles in a fluid medium.
- Stability: Colloids are generally stable and do not settle out on standing, unlike suspensions.
Applications of Colloids
Colloids are widely used in various industries and everyday products, including food (e.g., mayonnaise, milk), medicine (e.g., pharmaceutical formulations), and cosmetics (e.g., lotions, creams).
Study Guide
- What are the types of colloids? Explain each with an example.
- What are the properties of colloids?
- How are colloids different from solutions and suspensions?
- Provide examples of the application of colloids in everyday life.
[Colloids] Related Worksheets and Study Guides:
.◂Science Worksheets and Study Guides Seventh Grade. Cell Reproduction
Study Guide Cell Reproduction
Cell Reproduction  Activity Lesson
Activity Lesson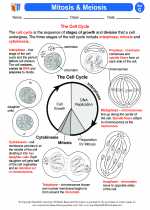 Mitosis & Meiosis
Mitosis & Meiosis  Worksheet/Answer key
Worksheet/Answer key Cell Reproduction
Cell Reproduction  Worksheet/Answer key
Worksheet/Answer key Cell Reproduction
Cell Reproduction  Worksheet/Answer key
Worksheet/Answer key Cell Reproduction
Cell Reproduction  Vocabulary/Answer key
Vocabulary/Answer key Cell Reproduction
Cell Reproduction  Vocabulary/Answer key
Vocabulary/Answer key Cell Reproduction
Cell Reproduction  Vocabulary/Answer key
Vocabulary/Answer key Cell Reproduction
Cell Reproduction  Vocabulary/Answer key
Vocabulary/Answer key Cell Reproduction
Cell Reproduction  Vocabulary/Answer key
Vocabulary/Answer key Cell Reproduction
Cell Reproduction  Vocabulary/Answer key
Vocabulary/Answer key Cell Reproduction
Cell Reproduction  Vocabulary/Answer key
Vocabulary/Answer key Cell Reproduction
Cell Reproduction  Vocabulary/Answer key
Vocabulary/Answer key Cell Reproduction
Cell Reproduction 

 Activity Lesson
Activity Lesson
 Worksheet/Answer key
Worksheet/Answer key
 Worksheet/Answer key
Worksheet/Answer key
 Worksheet/Answer key
Worksheet/Answer key
 Vocabulary/Answer key
Vocabulary/Answer key
 Vocabulary/Answer key
Vocabulary/Answer key
 Vocabulary/Answer key
Vocabulary/Answer key
 Vocabulary/Answer key
Vocabulary/Answer key
 Vocabulary/Answer key
Vocabulary/Answer key
 Vocabulary/Answer key
Vocabulary/Answer key
 Vocabulary/Answer key
Vocabulary/Answer key
 Vocabulary/Answer key
Vocabulary/Answer key

The resources above cover the following skills:
LIFE SCIENCE
From Molecules to Organisms: Structures and Processes
Gather and synthesize information to explain how prokaryotic and eukaryotic cells differ in structure and function, including the methods of asexual and sexual reproduction.