Universal Solvent
The term "universal solvent" refers to a substance that has the ability to dissolve a wide range of solutes. Water is often referred to as the universal solvent because of its unique properties that allow it to dissolve many different substances. This property makes water essential for life on Earth, as it can dissolve and transport essential nutrients and minerals.
Properties of Water as a Universal Solvent:
- Polarity: Water is a polar molecule, meaning it has a slightly positive end and a slightly negative end. This polarity allows water to interact with and dissolve other polar and ionic substances.
- Hydrogen Bonding: Water molecules can form hydrogen bonds with other polar molecules, which facilitates the dissolution of substances in water.
- Versatility: Water can dissolve a wide variety of solutes, including salts, sugars, acids, and gases.
Study Guide:
- Define the term "universal solvent" and provide an example.
- Explain the importance of water as a universal solvent in biological systems.
- Describe the properties of water that make it an effective universal solvent.
- Compare and contrast the solubility of polar and nonpolar substances in water.
- Discuss the role of universal solvents in environmental processes, such as weathering and erosion.
Understanding the concept of a universal solvent, specifically water, is crucial in the study of chemistry, biology, and environmental science. It is important to grasp the properties and significance of water as a solvent in various natural and artificial processes.
.◂Science Worksheets and Study Guides Seventh Grade. Cell Reproduction
Study Guide Cell Reproduction
Cell Reproduction  Activity Lesson
Activity Lesson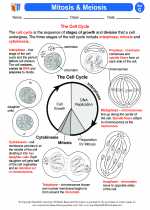 Mitosis & Meiosis
Mitosis & Meiosis  Worksheet/Answer key
Worksheet/Answer key Cell Reproduction
Cell Reproduction  Worksheet/Answer key
Worksheet/Answer key Cell Reproduction
Cell Reproduction  Worksheet/Answer key
Worksheet/Answer key Cell Reproduction
Cell Reproduction  Vocabulary/Answer key
Vocabulary/Answer key Cell Reproduction
Cell Reproduction  Vocabulary/Answer key
Vocabulary/Answer key Cell Reproduction
Cell Reproduction  Vocabulary/Answer key
Vocabulary/Answer key Cell Reproduction
Cell Reproduction  Vocabulary/Answer key
Vocabulary/Answer key Cell Reproduction
Cell Reproduction  Vocabulary/Answer key
Vocabulary/Answer key Cell Reproduction
Cell Reproduction  Vocabulary/Answer key
Vocabulary/Answer key Cell Reproduction
Cell Reproduction  Vocabulary/Answer key
Vocabulary/Answer key Cell Reproduction
Cell Reproduction  Vocabulary/Answer key
Vocabulary/Answer key Cell Reproduction
Cell Reproduction 

 Activity Lesson
Activity Lesson
 Worksheet/Answer key
Worksheet/Answer key
 Worksheet/Answer key
Worksheet/Answer key
 Worksheet/Answer key
Worksheet/Answer key
 Vocabulary/Answer key
Vocabulary/Answer key
 Vocabulary/Answer key
Vocabulary/Answer key
 Vocabulary/Answer key
Vocabulary/Answer key
 Vocabulary/Answer key
Vocabulary/Answer key
 Vocabulary/Answer key
Vocabulary/Answer key
 Vocabulary/Answer key
Vocabulary/Answer key
 Vocabulary/Answer key
Vocabulary/Answer key
 Vocabulary/Answer key
Vocabulary/Answer key

The resources above cover the following skills:
LIFE SCIENCE
From Molecules to Organisms: Structures and Processes
Gather and synthesize information to explain how prokaryotic and eukaryotic cells differ in structure and function, including the methods of asexual and sexual reproduction.