Freezing: An Overview
Freezing is the process in which a liquid turns into a solid when its temperature is lowered below its freezing point. This phase transition occurs when the kinetic energy of the molecules in the liquid decreases to the point where they are no longer able to overcome the attractive forces between them, leading to the formation of a solid structure. Freezing is an important phenomenon in everyday life and has numerous applications in various fields, including food preservation, cryopreservation, and the formation of snow and ice.
Key Concepts to Understand:
- Freezing Point: The temperature at which a substance changes from a liquid to a solid at a given pressure.
- Nucleation: The initial formation of a solid phase from a liquid phase, which can be spontaneous or induced by external factors.
- Crystallization: The process by which the molecules in a liquid arrange themselves in a highly ordered, repeating pattern to form a solid crystal lattice.
- Supercooling: The process in which a liquid is cooled below its freezing point without solidifying, often due to the absence of nucleation sites.
Factors Affecting Freezing:
Several factors can influence the freezing process, including temperature, pressure, impurities, and the presence of nucleation agents. Understanding these factors is crucial for predicting and controlling the freezing behavior of different substances.
Study Guide:
As you study the topic of freezing, it's important to focus on the following key areas:
- Understand the concept of freezing point and how it varies for different substances.
- Explore the role of nucleation in the freezing process and its significance in the formation of ice and other solid structures.
- Learn about the effects of impurities and pressure on the freezing point of a substance.
- Examine real-world applications of freezing, such as food preservation techniques and the use of cryoprotectants in cryopreservation.
- Consider the environmental and scientific implications of freezing, including its role in climate patterns, the formation of glaciers, and the study of materials science.
By mastering these concepts and principles, you will gain a comprehensive understanding of the phenomenon of freezing and its significance in the world around us.
.◂Science Worksheets and Study Guides Seventh Grade. Ecosystems, food chains and food webs

 Activity Lesson
Activity Lesson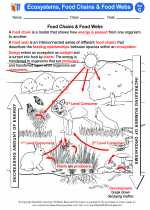
 Worksheet/Answer key
Worksheet/Answer key
 Worksheet/Answer key
Worksheet/Answer key
 Worksheet/Answer key
Worksheet/Answer key
 Vocabulary/Answer key
Vocabulary/Answer key
 Vocabulary/Answer key
Vocabulary/Answer key
 Vocabulary/Answer key
Vocabulary/Answer key
 Vocabulary/Answer key
Vocabulary/Answer key
 Vocabulary/Answer key
Vocabulary/Answer key
