Chromatography
Chromatography is a laboratory technique used to separate and identify the components of a mixture. It relies on the principle that different compounds in a mixture will interact differently with a stationary phase and a mobile phase, causing them to move at different rates and thus become separated.
Types of Chromatography
There are several types of chromatography, including:
- Thin Layer Chromatography (TLC): In TLC, a thin layer of adsorbent material is coated on a glass, metal, or plastic plate. The sample mixture is applied near the bottom of the plate and the plate is then placed in a solvent. As the solvent moves up the plate, it carries the sample mixture with it, and the components of the mixture separate based on their interactions with the adsorbent material.
- Gas Chromatography (GC): In GC, the sample is vaporized and injected into a long, coiled tube containing a stationary phase. A carrier gas (such as helium or nitrogen) carries the vaporized sample through the tube. The different components of the sample interact with the stationary phase at different rates, causing them to separate as they exit the tube.
- Liquid Chromatography (LC): In LC, the sample is dissolved in a liquid solvent and passed through a column containing a stationary phase. The components of the sample interact with the stationary phase and the solvent at different rates, causing them to separate as they flow through the column.
Applications of Chromatography
Chromatography has a wide range of applications in various fields, including:
- Drug testing and development
- Environmental analysis
- Forensic science
- Food and beverage industry
- Biochemical research
Study Guide
To understand chromatography better, it's important to focus on the following key points:
- Understand the principle of chromatography and the interactions between the stationary phase, mobile phase, and sample components.
- Be able to identify the different types of chromatography and explain the specific differences and applications of each type.
- Learn how to interpret chromatograms and understand how to calculate retention times and retention factors for separated components.
- Explore real-life examples and case studies where chromatography has been used to solve analytical problems or separate complex mixtures.
By mastering these key points, you will have a solid understanding of chromatography and its importance in scientific and analytical processes.
Good luck with your studies!
.◂Science Worksheets and Study Guides Eighth Grade. Plate tectonics
Study Guide Plate tectonics
Plate tectonics  Activity Lesson
Activity Lesson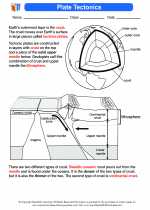 Plate Tectonics
Plate Tectonics  Worksheet/Answer key
Worksheet/Answer key Plate tectonics
Plate tectonics  Worksheet/Answer key
Worksheet/Answer key Plate tectonics
Plate tectonics  Worksheet/Answer key
Worksheet/Answer key Plate tectonics
Plate tectonics  Worksheet/Answer key
Worksheet/Answer key Plate tectonics
Plate tectonics  Vocabulary/Answer key
Vocabulary/Answer key Plate tectonics
Plate tectonics  Vocabulary/Answer key
Vocabulary/Answer key Plate tectonics
Plate tectonics  Vocabulary/Answer key
Vocabulary/Answer key Plate tectonics
Plate tectonics  Vocabulary/Answer key
Vocabulary/Answer key Plate tectonics
Plate tectonics  Vocabulary/Answer key
Vocabulary/Answer key Plate tectonics
Plate tectonics  Vocabulary/Answer key
Vocabulary/Answer key Plate tectonics
Plate tectonics  Vocabulary/Answer key
Vocabulary/Answer key Plate tectonics
Plate tectonics 

 Activity Lesson
Activity Lesson
 Worksheet/Answer key
Worksheet/Answer key
 Worksheet/Answer key
Worksheet/Answer key
 Worksheet/Answer key
Worksheet/Answer key
 Worksheet/Answer key
Worksheet/Answer key
 Vocabulary/Answer key
Vocabulary/Answer key
 Vocabulary/Answer key
Vocabulary/Answer key
 Vocabulary/Answer key
Vocabulary/Answer key
 Vocabulary/Answer key
Vocabulary/Answer key
 Vocabulary/Answer key
Vocabulary/Answer key
 Vocabulary/Answer key
Vocabulary/Answer key
 Vocabulary/Answer key
Vocabulary/Answer key
