Atom's Nucleus
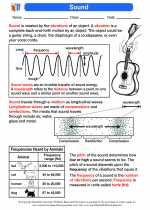
The nucleus is the central part of an atom, comprising protons and neutrons. It was discovered by Ernest Rutherford in 1911 through his gold foil experiment. The nucleus is tiny compared to the overall size of the atom but contains most of its mass. Understanding the structure and properties of the nucleus is essential in understanding the behavior of atoms and elements.
Structure of the Nucleus
The nucleus is composed of protons and neutrons, which are tightly bound together by the strong nuclear force. Protons have a positive charge, while neutrons have no charge. The number of protons in the nucleus determines the element's identity, while the number of neutrons can vary, leading to different isotopes of the same element.
Nuclear Forces
The strong nuclear force is the most powerful force in nature, holding the nucleus together. This force overcomes the repulsive electromagnetic force between protons, which would otherwise cause the nucleus to fly apart. The weak nuclear force is responsible for certain types of radioactive decay, such as beta decay.
Nuclear Reactions
Nuclear reactions involve changes in the composition of the nucleus, leading to the release of large amounts of energy. This energy is the basis for nuclear power and nuclear weapons. Understanding nuclear reactions is crucial for harnessing nuclear energy and ensuring its safe and responsible use.
Study Tips
- Review the structure and properties of protons and neutrons.
- Understand the concept of isotopes and how they relate to the nucleus.
- Learn about the forces that act within the nucleus, such as the strong nuclear force and weak nuclear force.
- Explore the different types of nuclear reactions and their implications.
- Practice solving problems related to nuclear stability, decay, and energy release.
By mastering the concepts related to the atom's nucleus, you will gain a deeper understanding of the fundamental building blocks of matter and their role in shaping the universe.
.