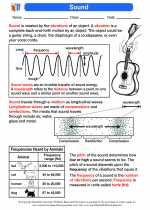
Boiling Point
The boiling point of a substance is the temperature at which the vapor pressure of the liquid equals the external pressure surrounding the liquid. This is the temperature at which a liquid turns into a gas throughout the entire liquid. The boiling point is a characteristic physical property of a substance and can be used to identify and classify different compounds.
Factors Affecting Boiling Point:
Several factors can affect the boiling point of a substance, including:
- Molecular weight: Generally, the higher the molecular weight of a substance, the higher its boiling point.
- Intermolecular forces: Substances with stronger intermolecular forces (such as hydrogen bonding) tend to have higher boiling points.
- Pressure: The external pressure can affect the boiling point of a substance. Higher pressures generally lead to higher boiling points.
Study Guide:
To understand the concept of boiling point, it's important to study the following topics:
- Intermolecular forces: Understand the different types of intermolecular forces such as hydrogen bonding, dipole-dipole interactions, and London dispersion forces.
- Phase transitions: Learn about the different phase transitions, including the processes of evaporation, boiling, and condensation.
- Boiling point calculations: Practice solving problems that involve calculating the boiling point of a substance based on its molecular properties and external pressure.
- Boiling point trends: Explore the trends in boiling points across different groups of compounds, such as alkanes, alcohols, and carboxylic acids.
Understanding the boiling point is crucial in various fields, including chemistry, biology, and environmental science. It is a fundamental concept that forms the basis for understanding the behavior of substances in different conditions.
.