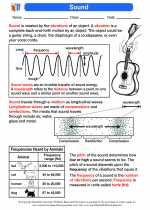
Combustion Reaction
A combustion reaction is a type of chemical reaction in which a substance combines with oxygen to produce heat, light, and new chemical compounds. This reaction is also known as burning, and it is an important process in various natural and industrial activities.
Key Concepts
- Reactants: The substances that undergo combustion, usually a fuel and oxygen.
- Products: The new substances formed as a result of the combustion reaction, such as carbon dioxide and water vapor.
- Energy Release: Combustion reactions release energy in the form of heat and light.
- Types of Combustion: Complete combustion and incomplete combustion.
- Applications: Combustion reactions are used in heating, cooking, and as a source of energy in engines.
Examples of Combustion Reactions
Some common examples of combustion reactions include:
- Combustion of methane: CH4 + 2O2 → CO2 + 2H2O
- Combustion of propane: C3H8 + 5O2 → 3CO2 + 4H2O
- Combustion of wood: C6H12O6 + 6O2 → 6CO2 + 6H2O
Study Guide
When studying combustion reactions, it is important to understand the following:
- Identify the reactants and products of a combustion reaction.
- Understand the role of oxygen in combustion reactions.
- Differentiate between complete combustion and incomplete combustion.
- Learn about the applications and implications of combustion reactions in daily life and industry.
- Practice balancing combustion reaction equations.
Remember to review the examples of combustion reactions and work on practice problems to reinforce your understanding of the topic.
.