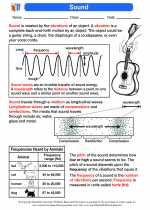
Crystal Lattice
A crystal lattice is a repeating three-dimensional pattern of particles, such as atoms, ions, or molecules, arranged in a highly ordered structure. This arrangement gives crystals their unique properties, such as distinct shapes, cleavage patterns, and optical properties.
Types of Crystal Lattices
There are several types of crystal lattices, including:
- Simple Cubic Lattice: In this type of lattice, the particles are arranged at the corners of a cube. It is the simplest and least dense type of lattice.
- Body-Centered Cubic Lattice: In this lattice, particles are present at the corners of the cube as well as at the center of the cube.
- Face-Centered Cubic Lattice: This lattice has particles at the corners of the cube and also at the center of each face of the cube.
- Hexagonal Close-Packed Lattice: In this lattice, the particles are arranged in a closely packed hexagonal pattern.
- Cubic Close-Packed Lattice: This lattice has a cubic structure with particles arranged in a closely packed manner.
Characteristics of Crystal Lattices
Key characteristics of crystal lattices include:
- Repetition: The arrangement of particles repeats in all three dimensions, creating a regular pattern throughout the crystal.
- Orderliness: The particles are arranged in a highly ordered manner, which contributes to the crystal's stability and distinct properties.
- Unit Cell: The smallest repeating unit of the crystal lattice is known as the unit cell, which reflects the overall symmetry and arrangement of the lattice.
- Coordination Number: Each particle in the lattice is in direct contact with a certain number of neighboring particles, known as its coordination number.
Study Guide for Crystal Lattice
To understand crystal lattices, students should focus on the following concepts:
- Definition of a crystal lattice and its significance in the formation of crystalline solids.
- Identification of different types of crystal lattices, including simple cubic, body-centered cubic, face-centered cubic, hexagonal close-packed, and cubic close-packed lattices.
- Understanding the concept of unit cells and their role in defining the structure of crystal lattices.
- Exploring the coordination number and its impact on the physical properties of crystals.
- Relating the arrangement of particles in crystal lattices to the macroscopic properties of crystals, such as cleavage patterns, optical behavior, and symmetry.