Cations
Definition:
A cation is a positively charged ion that is formed when an atom loses one or more electrons.
Formation of Cations:
When an atom loses one or more electrons, it becomes positively charged and forms a cation. This typically occurs in reactions where atoms undergo oxidation, releasing electrons in the process.
Naming Cations:
Cations are named by using the name of the element followed by the word "ion." For example, a sodium cation is simply called a sodium ion (Na+).
Properties of Cations:
- Cations are smaller in size compared to their parent atoms.
- They are attracted to the negative terminal (cathode) in electrolysis.
- Cations are often metals and tend to form ionic compounds with anions (negatively charged ions).
Examples of Common Cations:
Some common cations include:
Study Guide:
Here are some key points to remember about cations:
- Understand the concept of ion formation and the role of electrons in forming cations.
- Memorize the names and charges of common cations.
- Practice writing chemical formulas and names for cations and anions.
- Learn the properties and behavior of cations in chemical reactions and electrolysis.
◂Science Worksheets and Study Guides Eighth Grade. The nervous system
Study Guide The nervous system
The nervous system  Worksheet/Answer key
Worksheet/Answer key The nervous system
The nervous system  Worksheet/Answer key
Worksheet/Answer key The nervous system
The nervous system  Worksheet/Answer key
Worksheet/Answer key The nervous system
The nervous system  Vocabulary/Answer key
Vocabulary/Answer key The nervous system
The nervous system  Vocabulary/Answer key
Vocabulary/Answer key The nervous system
The nervous system  Vocabulary/Answer key
Vocabulary/Answer key The nervous system
The nervous system  Vocabulary/Answer key
Vocabulary/Answer key The nervous system
The nervous system  Vocabulary/Answer key
Vocabulary/Answer key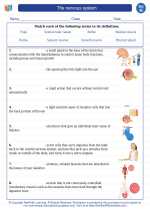 The nervous system
The nervous system  Vocabulary/Answer key
Vocabulary/Answer key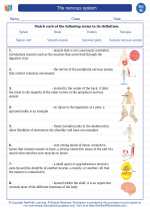 The nervous system
The nervous system 

 Worksheet/Answer key
Worksheet/Answer key
 Worksheet/Answer key
Worksheet/Answer key
 Worksheet/Answer key
Worksheet/Answer key
 Vocabulary/Answer key
Vocabulary/Answer key
 Vocabulary/Answer key
Vocabulary/Answer key
 Vocabulary/Answer key
Vocabulary/Answer key
 Vocabulary/Answer key
Vocabulary/Answer key
 Vocabulary/Answer key
Vocabulary/Answer key
 Vocabulary/Answer key
Vocabulary/Answer key
