High Specific Heat
Specific heat is the amount of heat energy required to raise the temperature of a substance by a certain amount. When a substance has a high specific heat, it means that it can absorb a large amount of heat energy without its temperature increasing significantly.
Water is a great example of a substance with a high specific heat. This is because it takes a lot of energy to raise the temperature of water, and it can also release a lot of energy when it cools down.
Study Guide
- Definition: Specific heat is the amount of heat energy required to raise the temperature of a substance by a certain amount. A high specific heat means that a substance can absorb a large amount of heat energy without its temperature increasing significantly.
- Examples: Water is a common example of a substance with a high specific heat. Other substances with high specific heat include ammonia and liquid hydrogen.
- Importance: High specific heat is important for regulating temperature in organisms and the environment. It helps to stabilize temperature in bodies of water and living organisms, preventing rapid temperature changes that could be harmful.
- Measurement: The specific heat of a substance is typically measured in joules per gram degree Celsius (J/g°C) or calories per gram degree Celsius (cal/g°C).
- Applications: Understanding the concept of high specific heat is important in fields such as biology, environmental science, and engineering. It is also relevant in everyday life, such as in cooking and heating systems.
Understanding the concept of high specific heat and its significance can provide insights into various natural and industrial processes, as well as help in the development of technologies that rely on heat transfer and temperature regulation.
.◂Science Worksheets and Study Guides Kindergarten. All About Animals
Coloring Worksheet Birds
Birds  Coloring Worksheet
Coloring Worksheet Birds
Birds  Coloring Worksheet
Coloring Worksheet Bugs
Bugs  Coloring Worksheet
Coloring Worksheet Bugs
Bugs  Coloring Worksheet
Coloring Worksheet Butterfly life cycle
Butterfly life cycle  Coloring Worksheet
Coloring Worksheet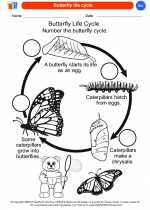 Butterfly life cycle
Butterfly life cycle  Coloring Worksheet
Coloring Worksheet Farm & Zoo
Farm & Zoo  Coloring Worksheet
Coloring Worksheet Farm & Zoo
Farm & Zoo  Coloring Worksheet
Coloring Worksheet Frog life cycle
Frog life cycle  Coloring Worksheet
Coloring Worksheet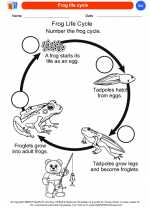 Frog life cycle
Frog life cycle  Coloring Worksheet
Coloring Worksheet Interdependence
Interdependence  Coloring Worksheet
Coloring Worksheet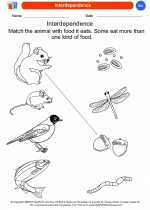 Interdependence
Interdependence  Coloring Worksheet
Coloring Worksheet Offspring
Offspring  Coloring Worksheet
Coloring Worksheet Offspring
Offspring  Coloring Worksheet
Coloring Worksheet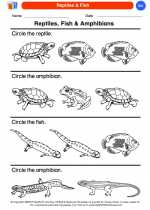 Reptiles & Fish
Reptiles & Fish  Coloring Worksheet
Coloring Worksheet Reptiles & Fish
Reptiles & Fish  Coloring Worksheet
Coloring Worksheet What animals need
What animals need  Coloring Worksheet
Coloring Worksheet What animals need
What animals need 

 Coloring Worksheet
Coloring Worksheet
 Coloring Worksheet
Coloring Worksheet
 Coloring Worksheet
Coloring Worksheet
 Coloring Worksheet
Coloring Worksheet
 Coloring Worksheet
Coloring Worksheet
 Coloring Worksheet
Coloring Worksheet
 Coloring Worksheet
Coloring Worksheet
 Coloring Worksheet
Coloring Worksheet
 Coloring Worksheet
Coloring Worksheet
 Coloring Worksheet
Coloring Worksheet
 Coloring Worksheet
Coloring Worksheet
 Coloring Worksheet
Coloring Worksheet
 Coloring Worksheet
Coloring Worksheet
 Coloring Worksheet
Coloring Worksheet
 Coloring Worksheet
Coloring Worksheet
 Coloring Worksheet
Coloring Worksheet
 Coloring Worksheet
Coloring Worksheet

The resources above cover the following skills:
Science, Kindergarten, Adopted 2017 – The provisions of §§112.11-112.16 of this subchapter shall be implemented by school districts beginning with the 2018-2019 school year.
Knowledge and skills.
Organisms and environments. The student knows that organisms resemble their parents and have structures and processes that help them survive within their environments. The student is expected to:
sort plants and animals into groups based on physical characteristics such as color, size, body covering, or leaf shape
identify basic parts of plants and animals