Electrons
Electrons are subatomic particles that are found in the electron cloud surrounding an atom's nucleus. They have a negative charge and are incredibly small in comparison to protons and neutrons. Understanding the behavior and properties of electrons is vital to understanding the fundamental principles of chemistry and physics.
Key Points to Remember:
- Electrons are negatively charged subatomic particles.
- They are found in the electron cloud surrounding the nucleus of an atom.
- Electrons are involved in the formation of chemical bonds and the transfer of electricity.
- Electrons have a much smaller mass compared to protons and neutrons.
- Electrons exhibit wave-particle duality, behaving both as particles and waves.
Study Guide:
Here are some key concepts to focus on when studying electrons:
- Atomic Structure: Understand the arrangement of electrons within an atom, including energy levels and electron configuration.
- Electron Orbitals: Learn about the different shapes and orientations of electron orbitals within an atom.
- Electron Configuration: Practice writing electron configurations for different elements and understanding the principles behind the Aufbau principle, Pauli exclusion principle, and Hund's rule.
- Chemical Bonding: Explore how electrons are involved in the formation of chemical bonds, including covalent, ionic, and metallic bonding.
- Electricity and Conductivity: Investigate the role of electrons in the flow of electricity and the concept of electrical conductivity.
By mastering these concepts, you will develop a solid understanding of the crucial role that electrons play in the world of science and technology.
.◂Science Worksheets and Study Guides Kindergarten. Pushing, Moving, Pulling
Coloring Worksheet How heavy
How heavy  Coloring Worksheet
Coloring Worksheet How heavy
How heavy  Coloring Worksheet
Coloring Worksheet How Things Move
How Things Move  Coloring Worksheet
Coloring Worksheet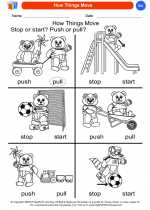 How Things Move
How Things Move  Coloring Worksheet
Coloring Worksheet Light and Heat
Light and Heat  Coloring Worksheet
Coloring Worksheet Light and Heat
Light and Heat  Coloring Worksheet
Coloring Worksheet Magnets
Magnets  Coloring Worksheet
Coloring Worksheet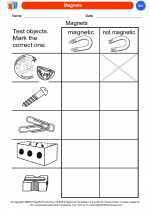 Magnets
Magnets  Coloring Worksheet
Coloring Worksheet Pushing and Pulling
Pushing and Pulling  Coloring Worksheet
Coloring Worksheet Pushing and Pulling
Pushing and Pulling  Coloring Worksheet
Coloring Worksheet Simple Machines
Simple Machines  Coloring Worksheet
Coloring Worksheet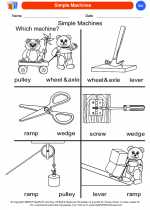 Simple Machines
Simple Machines  Coloring Worksheet
Coloring Worksheet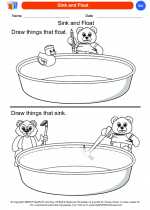 Sink and Float
Sink and Float  Coloring Worksheet
Coloring Worksheet Sink and Float
Sink and Float  Coloring Worksheet
Coloring Worksheet Sound All Around
Sound All Around  Coloring Worksheet
Coloring Worksheet Sound All Around
Sound All Around  Coloring Worksheet
Coloring Worksheet Up and Down
Up and Down  Coloring Worksheet
Coloring Worksheet Up and Down
Up and Down  Coloring Worksheet
Coloring Worksheet Wheels
Wheels  Coloring Worksheet
Coloring Worksheet Wheels
Wheels 

 Coloring Worksheet
Coloring Worksheet
 Coloring Worksheet
Coloring Worksheet
 Coloring Worksheet
Coloring Worksheet
 Coloring Worksheet
Coloring Worksheet
 Coloring Worksheet
Coloring Worksheet
 Coloring Worksheet
Coloring Worksheet
 Coloring Worksheet
Coloring Worksheet
 Coloring Worksheet
Coloring Worksheet
 Coloring Worksheet
Coloring Worksheet
 Coloring Worksheet
Coloring Worksheet
 Coloring Worksheet
Coloring Worksheet
 Coloring Worksheet
Coloring Worksheet
 Coloring Worksheet
Coloring Worksheet
 Coloring Worksheet
Coloring Worksheet
 Coloring Worksheet
Coloring Worksheet
 Coloring Worksheet
Coloring Worksheet
 Coloring Worksheet
Coloring Worksheet
 Coloring Worksheet
Coloring Worksheet
 Coloring Worksheet
Coloring Worksheet
