Key Points about Protons:
- Protons are positively charged subatomic particles.
- They are found in the nucleus of an atom.
- They have a relative mass of 1.
- The number of protons in an atom's nucleus determines the element's identity and is known as the atomic number.
Study Guide for Protons:
Here are some key concepts to understand about protons:
- Properties of Protons: Understand the basic properties of protons, including their charge, location, and relative mass.
- Atomic Number: Learn how the number of protons in an atom's nucleus determines the identity of the element.
- Role in Chemistry: Understand the role of protons in chemical reactions and bonding.
- Isotopes: Explore the concept of isotopes, which have the same number of protons but different numbers of neutrons.
- Experimental Methods: Learn about experimental methods used to study protons, such as particle accelerators and spectroscopy.
Interactive Activities:
Engage in hands-on activities to deepen your understanding of protons:
- Modeling Atomic Structures: Use materials to create models of atoms, highlighting the location and role of protons.
- Element Identification: Practice identifying elements based on their atomic number and the number of protons.
- Virtual Experiments: Explore online simulations and virtual labs to observe the behavior of protons in different contexts.
◂Science Worksheets and Study Guides Kindergarten. Pushing, Moving, Pulling
Coloring Worksheet How heavy
How heavy  Coloring Worksheet
Coloring Worksheet How heavy
How heavy  Coloring Worksheet
Coloring Worksheet How Things Move
How Things Move  Coloring Worksheet
Coloring Worksheet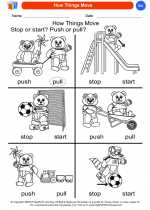 How Things Move
How Things Move  Coloring Worksheet
Coloring Worksheet Light and Heat
Light and Heat  Coloring Worksheet
Coloring Worksheet Light and Heat
Light and Heat  Coloring Worksheet
Coloring Worksheet Magnets
Magnets  Coloring Worksheet
Coloring Worksheet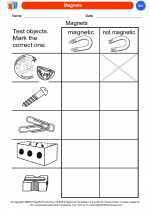 Magnets
Magnets  Coloring Worksheet
Coloring Worksheet Pushing and Pulling
Pushing and Pulling  Coloring Worksheet
Coloring Worksheet Pushing and Pulling
Pushing and Pulling  Coloring Worksheet
Coloring Worksheet Simple Machines
Simple Machines  Coloring Worksheet
Coloring Worksheet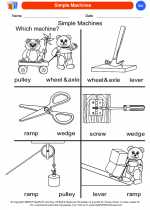 Simple Machines
Simple Machines  Coloring Worksheet
Coloring Worksheet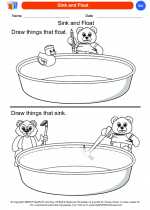 Sink and Float
Sink and Float  Coloring Worksheet
Coloring Worksheet Sink and Float
Sink and Float  Coloring Worksheet
Coloring Worksheet Sound All Around
Sound All Around  Coloring Worksheet
Coloring Worksheet Sound All Around
Sound All Around  Coloring Worksheet
Coloring Worksheet Up and Down
Up and Down  Coloring Worksheet
Coloring Worksheet Up and Down
Up and Down  Coloring Worksheet
Coloring Worksheet Wheels
Wheels  Coloring Worksheet
Coloring Worksheet Wheels
Wheels 

 Coloring Worksheet
Coloring Worksheet
 Coloring Worksheet
Coloring Worksheet
 Coloring Worksheet
Coloring Worksheet
 Coloring Worksheet
Coloring Worksheet
 Coloring Worksheet
Coloring Worksheet
 Coloring Worksheet
Coloring Worksheet
 Coloring Worksheet
Coloring Worksheet
 Coloring Worksheet
Coloring Worksheet
 Coloring Worksheet
Coloring Worksheet
 Coloring Worksheet
Coloring Worksheet
 Coloring Worksheet
Coloring Worksheet
 Coloring Worksheet
Coloring Worksheet
 Coloring Worksheet
Coloring Worksheet
 Coloring Worksheet
Coloring Worksheet
 Coloring Worksheet
Coloring Worksheet
 Coloring Worksheet
Coloring Worksheet
 Coloring Worksheet
Coloring Worksheet
 Coloring Worksheet
Coloring Worksheet
 Coloring Worksheet
Coloring Worksheet
