Atomic Number
The atomic number of an element is the number of protons found in the nucleus of an atom of that element. It is a unique identifier for each element on the periodic table. The atomic number determines the chemical properties of the element and its place in the periodic table.
Key Points to Remember
- The atomic number is represented by the symbol "Z".
- Elements are arranged in increasing order of their atomic numbers in the periodic table.
- The number of protons also determines the identity of the element. For example, all carbon atoms have 6 protons in their nucleus, so the atomic number of carbon is 6.
- The atomic number can be used to determine the number of electrons in a neutral atom of the element. In a neutral atom, the number of electrons is equal to the number of protons.
Study Tips
Here are some tips to help you remember and understand the concept of atomic number:
- Memorize the atomic numbers of common elements such as hydrogen (1), oxygen (8), carbon (6), and nitrogen (7).
- Understand that elements with the same atomic number are isotopes, meaning they have the same number of protons but different numbers of neutrons.
- Practice using the periodic table to locate elements and their atomic numbers.
- Make use of flashcards to quiz yourself on atomic numbers and their corresponding elements.
By mastering the concept of atomic number, you will have a solid foundation for understanding the properties and behavior of different elements in chemistry.
[Atomic Number] Related Worksheets and Study Guides:
.◂Science Worksheets and Study Guides Kindergarten. Pushing, Moving, Pulling
Coloring Worksheet How heavy
How heavy  Coloring Worksheet
Coloring Worksheet How heavy
How heavy  Coloring Worksheet
Coloring Worksheet How Things Move
How Things Move  Coloring Worksheet
Coloring Worksheet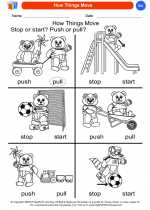 How Things Move
How Things Move  Coloring Worksheet
Coloring Worksheet Light and Heat
Light and Heat  Coloring Worksheet
Coloring Worksheet Light and Heat
Light and Heat  Coloring Worksheet
Coloring Worksheet Magnets
Magnets  Coloring Worksheet
Coloring Worksheet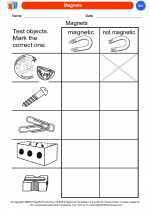 Magnets
Magnets  Coloring Worksheet
Coloring Worksheet Pushing and Pulling
Pushing and Pulling  Coloring Worksheet
Coloring Worksheet Pushing and Pulling
Pushing and Pulling  Coloring Worksheet
Coloring Worksheet Simple Machines
Simple Machines  Coloring Worksheet
Coloring Worksheet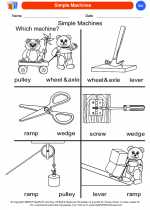 Simple Machines
Simple Machines  Coloring Worksheet
Coloring Worksheet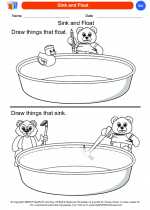 Sink and Float
Sink and Float  Coloring Worksheet
Coloring Worksheet Sink and Float
Sink and Float  Coloring Worksheet
Coloring Worksheet Sound All Around
Sound All Around  Coloring Worksheet
Coloring Worksheet Sound All Around
Sound All Around  Coloring Worksheet
Coloring Worksheet Up and Down
Up and Down  Coloring Worksheet
Coloring Worksheet Up and Down
Up and Down  Coloring Worksheet
Coloring Worksheet Wheels
Wheels  Coloring Worksheet
Coloring Worksheet Wheels
Wheels 

 Coloring Worksheet
Coloring Worksheet
 Coloring Worksheet
Coloring Worksheet
 Coloring Worksheet
Coloring Worksheet
 Coloring Worksheet
Coloring Worksheet
 Coloring Worksheet
Coloring Worksheet
 Coloring Worksheet
Coloring Worksheet
 Coloring Worksheet
Coloring Worksheet
 Coloring Worksheet
Coloring Worksheet
 Coloring Worksheet
Coloring Worksheet
 Coloring Worksheet
Coloring Worksheet
 Coloring Worksheet
Coloring Worksheet
 Coloring Worksheet
Coloring Worksheet
 Coloring Worksheet
Coloring Worksheet
 Coloring Worksheet
Coloring Worksheet
 Coloring Worksheet
Coloring Worksheet
 Coloring Worksheet
Coloring Worksheet
 Coloring Worksheet
Coloring Worksheet
 Coloring Worksheet
Coloring Worksheet
 Coloring Worksheet
Coloring Worksheet
