What is an Atom?
An atom is the basic building block of all matter. It is the smallest unit of an element that retains the chemical properties of that element. Atoms are made up of three subatomic particles: protons, neutrons, and electrons.
Structure of an Atom
The nucleus of an atom contains protons and neutrons, while electrons orbit the nucleus in energy levels or shells.
Protons: Positively charged particles found in the nucleus of an atom.
Neutrons: Neutral particles found in the nucleus of an atom.
Electrons: Negatively charged particles that orbit the nucleus in energy levels or shells.
Study Guide
- What are the three subatomic particles that make up an atom?
- Where are protons and neutrons located within an atom?
- Where are electrons located within an atom?
- What is the overall charge of an atom?
- What is the atomic number of an element?
- Explain the concept of energy levels or shells in an atom.
- How does the number of protons in an atom relate to its identity as a specific element?
- What is an ion?
- Describe the differences between an atom and an ion.
- Why is the study of atoms important in the field of chemistry?
[Atom] Related Worksheets and Study Guides:
.◂Science Worksheets and Study Guides Kindergarten. Weather
Coloring Worksheet Calendar
Calendar  Coloring Worksheet
Coloring Worksheet Calendar
Calendar  Coloring Worksheet
Coloring Worksheet Day and Night
Day and Night  Coloring Worksheet
Coloring Worksheet Day and Night
Day and Night  Coloring Worksheet
Coloring Worksheet Exploring Weather
Exploring Weather  Coloring Worksheet
Coloring Worksheet Exploring Weather
Exploring Weather  Coloring Worksheet
Coloring Worksheet Look at the Clouds
Look at the Clouds  Coloring Worksheet
Coloring Worksheet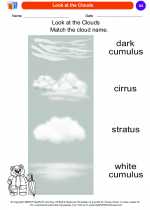 Look at the Clouds
Look at the Clouds  Coloring Worksheet
Coloring Worksheet Moon & Stars
Moon & Stars  Coloring Worksheet
Coloring Worksheet Moon & Stars
Moon & Stars  Coloring Worksheet
Coloring Worksheet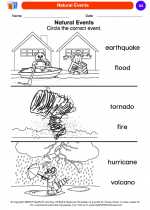 Natural Events
Natural Events  Coloring Worksheet
Coloring Worksheet Natural Events
Natural Events  Coloring Worksheet
Coloring Worksheet Sun and Shadows
Sun and Shadows  Coloring Worksheet
Coloring Worksheet Sun and Shadows
Sun and Shadows  Coloring Worksheet
Coloring Worksheet The Seasons
The Seasons  Coloring Worksheet
Coloring Worksheet The Seasons
The Seasons  Coloring Worksheet
Coloring Worksheet Things in the Sky
Things in the Sky  Coloring Worksheet
Coloring Worksheet Things in the Sky
Things in the Sky  Coloring Worksheet
Coloring Worksheet Weather Tools
Weather Tools  Coloring Worksheet
Coloring Worksheet Weather Tools
Weather Tools 

 Coloring Worksheet
Coloring Worksheet
 Coloring Worksheet
Coloring Worksheet
 Coloring Worksheet
Coloring Worksheet
 Coloring Worksheet
Coloring Worksheet
 Coloring Worksheet
Coloring Worksheet
 Coloring Worksheet
Coloring Worksheet
 Coloring Worksheet
Coloring Worksheet
 Coloring Worksheet
Coloring Worksheet
 Coloring Worksheet
Coloring Worksheet
 Coloring Worksheet
Coloring Worksheet
 Coloring Worksheet
Coloring Worksheet
 Coloring Worksheet
Coloring Worksheet
 Coloring Worksheet
Coloring Worksheet
 Coloring Worksheet
Coloring Worksheet
 Coloring Worksheet
Coloring Worksheet
 Coloring Worksheet
Coloring Worksheet
 Coloring Worksheet
Coloring Worksheet
 Coloring Worksheet
Coloring Worksheet
 Coloring Worksheet
Coloring Worksheet

The resources above cover the following skills:
EARTH AND SPACE SCIENCE (NGSS)
Earth’s Systems
Students who demonstrate understanding can:
Use and share observations of local weather conditions to describe patterns over time.