Aluminum
Aluminum is a chemical element with the symbol Al and atomic number 13. It is a silvery-white, soft, nonmagnetic, ductile metal in the boron group. By mass, aluminum makes up about 8% of the Earth's crust, making it the third most abundant element after oxygen and silicon.
Physical Properties:
- Melting Point: 660.32°C
- Boiling Point: 2519°C
- Density: 2.70 g/cm³
- Conductivity: Aluminum is a good conductor of heat and electricity.
Chemical Properties:
- Aluminum is highly reactive and can form a thin layer of oxide that protects it from corrosion.
- It reacts with both acids and bases.
- Aluminum does not easily corrode in water, as it forms a thin layer of aluminum oxide that protects the metal from further oxidation.
Uses of Aluminum:
Aluminum is widely used in various applications due to its advantageous properties, such as:
- Construction: Aluminum is used in the construction of buildings, infrastructure, and transportation vehicles due to its lightweight and corrosion-resistant properties.
- Packaging: Aluminum foil and containers are commonly used for packaging food and beverages due to their ability to protect the contents from light, oxygen, and moisture.
- Electrical transmission lines: Aluminum is used in overhead power lines due to its high conductivity and low cost.
- Aircraft and aerospace applications: The lightweight and strength of aluminum make it an ideal material for aircraft and aerospace components.
Study Guide:
Here are some study questions to help you understand the topic "aluminum":
- What is the symbol and atomic number of aluminum?
- What are the physical properties of aluminum?
- Describe the chemical properties of aluminum.
- What are some common uses of aluminum?
- Explain why aluminum is widely used in the aerospace industry.
By studying these questions, you can gain a comprehensive understanding of the properties and uses of aluminum.
.◂Science Worksheets and Study Guides First Grade. Living and nonliving things
Study Guide Living and nonliving things
Living and nonliving things  Activity Lesson
Activity Lesson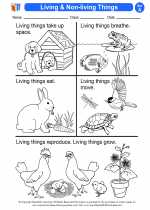 Living & Non-living Things
Living & Non-living Things  Worksheet/Answer key
Worksheet/Answer key Living and nonliving things
Living and nonliving things  Worksheet/Answer key
Worksheet/Answer key Living and nonliving things
Living and nonliving things  Worksheet/Answer key
Worksheet/Answer key Living and nonliving things
Living and nonliving things  Worksheet/Answer key
Worksheet/Answer key Living and nonliving things
Living and nonliving things  Vocabulary/Answer key
Vocabulary/Answer key Living and nonliving things
Living and nonliving things 

 Activity Lesson
Activity Lesson
 Worksheet/Answer key
Worksheet/Answer key
 Worksheet/Answer key
Worksheet/Answer key
 Worksheet/Answer key
Worksheet/Answer key
 Worksheet/Answer key
Worksheet/Answer key
 Vocabulary/Answer key
Vocabulary/Answer key

The resources above cover the following skills:
Concepts of Life Science (SC1, SC2, SC3)
The student demonstrates an understanding that all organisms are linked to each other and their physical environments through the transfer and transformation of matter and energy by identifying and sorting examples of living and non-living things in the local environment. (L)