Molecules
A molecule is a group of two or more atoms that are held together by chemical bonds. These atoms can be of the same element, as in the case of diatomic molecules like oxygen (O2) and hydrogen (H2), or they can be different elements, as in the case of water (H2O) and carbon dioxide (CO2).
Types of Molecules
There are two main types of molecules:
- Covalent Molecules: These are formed when atoms share electrons to form chemical bonds. Examples include water (H2O), carbon dioxide (CO2), and methane (CH4).
- Ionic Molecules: These are formed when atoms transfer electrons to form positive and negative ions, which then attract each other to form a molecule. Examples include sodium chloride (NaCl) and magnesium oxide (MgO).
Molecular Structure
The structure of a molecule refers to the arrangement of its atoms and the bonds between them. This structure determines the molecule's properties and behavior. For example, the arrangement of atoms in a water molecule gives it its unique properties, such as its ability to form hydrogen bonds and its role as a universal solvent.
Properties of Molecules
The properties of a molecule are determined by various factors, including its size, shape, and the types of atoms it contains. These properties influence how the molecule interacts with other substances and its behavior under different conditions, such as temperature and pressure.
Study Tips
Here are some tips for studying molecules:
- Understand the concept of chemical bonding and how atoms combine to form molecules.
- Learn to identify different types of molecules and their properties.
- Practice drawing molecular structures and understanding their three-dimensional shapes.
- Explore the role of molecules in everyday life, such as in the human body, food, and the environment.
- Use models and visual aids to visualize the structure and behavior of molecules.
◂Science Worksheets and Study Guides Fourth Grade. Organ systems

 Worksheet/Answer key
Worksheet/Answer key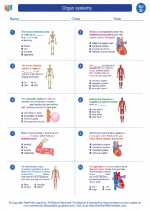
 Worksheet/Answer key
Worksheet/Answer key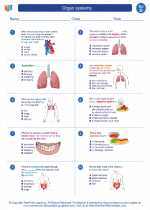
 Worksheet/Answer key
Worksheet/Answer key
 Vocabulary/Answer key
Vocabulary/Answer key
 Vocabulary/Answer key
Vocabulary/Answer key
 Vocabulary/Answer key
Vocabulary/Answer key
 Vocabulary/Answer key
Vocabulary/Answer key
 Vocabulary/Answer key
Vocabulary/Answer key
