Metal
Metal is a type of element that is typically hard, shiny, malleable, ductile, and a good conductor of heat and electricity. It makes up a large portion of the periodic table and is widely used in various industries and everyday objects.
Physical Properties of Metals
- Shiny: Metals have a characteristic luster or shine.
- Hardness: Most metals are hard, although some like sodium and potassium are soft.
- Malleability: Metals can be hammered or rolled into thin sheets without breaking.
- Ductility: Metals can be drawn into wires without breaking.
- Conductivity: Metals are good conductors of heat and electricity.
- High Melting and Boiling Points: Most metals have high melting and boiling points.
Chemical Properties of Metals
- Reactivity: Metals react with acids, oxygen, and other elements to form compounds.
- Corrosion: Some metals corrode when exposed to air and moisture, forming oxides or other compounds.
- Alloys: Metals can be combined with other elements to form alloys with enhanced properties.
Uses of Metals
Metals are used in a wide range of applications, including:
- Construction
- Transportation
- Electronics
- Medicine
- Cookware
- Jewelry
Study Guide
Here are some key points to remember when studying about metals:
- What are the physical properties of metals?
- How do metals react with other elements?
- What are some common uses of metals in everyday life?
- Can you name some examples of metal alloys and their uses?
Understanding the properties and uses of metals is important for various scientific and practical applications. Remember to review and practice examples to reinforce your understanding.
[Metal] Related Worksheets and Study Guides:
.◂Science Worksheets and Study Guides Fifth Grade. Flowers and seeds
Study Guide Flowers and seeds
Flowers and seeds  Activity Lesson
Activity Lesson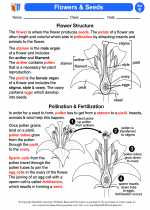 Flowers & Seeds
Flowers & Seeds  Worksheet/Answer key
Worksheet/Answer key Flowers and seeds
Flowers and seeds  Worksheet/Answer key
Worksheet/Answer key Flowers and seeds
Flowers and seeds  Worksheet/Answer key
Worksheet/Answer key Flowers and seeds
Flowers and seeds  Worksheet/Answer key
Worksheet/Answer key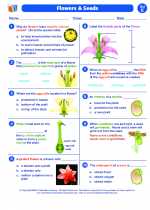 Flowers and seeds
Flowers and seeds  Vocabulary/Answer key
Vocabulary/Answer key Flowers and seeds
Flowers and seeds  Vocabulary/Answer key
Vocabulary/Answer key Flowers and seeds
Flowers and seeds 

 Activity Lesson
Activity Lesson
 Worksheet/Answer key
Worksheet/Answer key
 Worksheet/Answer key
Worksheet/Answer key
 Worksheet/Answer key
Worksheet/Answer key
 Worksheet/Answer key
Worksheet/Answer key
 Vocabulary/Answer key
Vocabulary/Answer key
 Vocabulary/Answer key
Vocabulary/Answer key

The resources above cover the following skills:
Life Science
All organisms have structures and systems with separate functions. Students can:
Develop and communicate an evidence-based scientific explanation of the role of different organs or structures that are important for an organism's survival - in both plants and animals