Sodium
Sodium is a chemical element with the symbol Na and atomic number 11. It is a soft, silvery-white, highly reactive metal and is a member of the alkali metal group of the periodic table. Sodium is an essential element for human health and is found in many common compounds, including table salt (sodium chloride).
Properties of Sodium
- Physical properties: Sodium is a soft and shiny metal that is easily cut with a knife. It has a low melting point and boiling point.
- Chemical properties: Sodium is highly reactive and readily forms compounds with other elements. It reacts violently with water, producing hydrogen gas and sodium hydroxide.
- Occurrence: Sodium is not found free in nature, but it occurs in many minerals, including halite (rock salt) and soda ash.
Uses of Sodium
Sodium has numerous industrial and biological applications:
- It is used in the production of numerous chemicals, including sodium hydroxide (caustic soda) and sodium carbonate (soda ash).
- It is used in the manufacture of soaps and detergents.
- Sodium compounds are used in the food industry as preservatives and flavor enhancers.
- Sodium vapor lamps are used for street lighting.
- It plays a crucial role in the functioning of nerves and muscles in the human body.
Study Guide
To study sodium, consider the following key points:
- Understand the properties of sodium, including its physical and chemical properties.
- Learn about the occurrence of sodium in nature and its extraction from minerals.
- Explore the various uses of sodium in industry and everyday life.
- Examine the biological importance of sodium in the human body.
- Review common compounds of sodium, such as sodium chloride and sodium hydroxide.
Understanding the role and significance of sodium will not only enhance your knowledge of chemistry but also provide insights into its importance in various fields.
[Sodium] Related Worksheets and Study Guides:
.◂Science Worksheets and Study Guides Sixth Grade. Magnetism
Study Guide Magnetism
Magnetism  Activity Lesson
Activity Lesson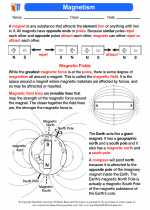 Magnetism
Magnetism  Worksheet/Answer key
Worksheet/Answer key Magnetism
Magnetism  Worksheet/Answer key
Worksheet/Answer key Magnetism
Magnetism  Worksheet/Answer key
Worksheet/Answer key Magnetism
Magnetism  Worksheet/Answer key
Worksheet/Answer key Magnetism
Magnetism  Vocabulary/Answer key
Vocabulary/Answer key Magnetism
Magnetism 

 Activity Lesson
Activity Lesson
 Worksheet/Answer key
Worksheet/Answer key
 Worksheet/Answer key
Worksheet/Answer key
 Worksheet/Answer key
Worksheet/Answer key
 Worksheet/Answer key
Worksheet/Answer key
 Vocabulary/Answer key
Vocabulary/Answer key

The resources above cover the following skills:
EARTH AND SPACE SCIENCE
Earth’s Systems
Develop and use models of Earth’s interior composition to illustrate the resulting magnetic field (e.g., magnetic poles) and to explain its measureable effects (e.g., protection from cosmic radiation).