Sublimation
Sublimation is the process in which a substance transitions directly from its solid phase to its gas phase without passing through the intermediate liquid phase. This occurs when the atmospheric pressure is lower than the substance's vapor pressure at a certain temperature. In simpler terms, sublimation is the conversion of a solid directly to a gas without first becoming a liquid.
Key Concepts:
- Phase Transition: Sublimation is a type of phase transition, which is a change in the physical state of a substance.
- Examples: Common examples of substances that undergo sublimation include dry ice (solid carbon dioxide) and solid iodine.
- Endothermic Process: Sublimation is an endothermic process, meaning it requires an input of energy to break the intermolecular forces holding the solid together.
- Reverse Process: The reverse of sublimation, where a gas transitions directly to a solid, is called deposition.
Study Guide:
Here are some key points to remember when studying sublimation:
- Understand the difference between sublimation and other phase transitions like melting, freezing, evaporation, and condensation.
- Be able to identify substances that undergo sublimation in everyday life.
- Know the factors that influence sublimation, such as temperature and pressure.
- Understand the concept of vapor pressure and how it relates to sublimation.
- Be familiar with the uses of sublimation in various fields, such as the production of dry ice and the purification of substances.
Remember to review the concept and practice identifying examples of sublimation to solidify your understanding.
With this study guide, you'll be well-prepared to understand and explain the process of sublimation.
.◂Science Worksheets and Study Guides Seventh Grade. Ecosystems, food chains and food webs
Study Guide Ecosystems, food chains and food webs
Ecosystems, food chains and food webs  Activity Lesson
Activity Lesson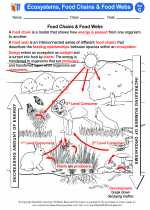 Ecosystems, Food Chains & Food Webs
Ecosystems, Food Chains & Food Webs  Worksheet/Answer key
Worksheet/Answer key Ecosystems, food chains and food webs
Ecosystems, food chains and food webs  Worksheet/Answer key
Worksheet/Answer key Ecosystems, food chains and food webs
Ecosystems, food chains and food webs  Worksheet/Answer key
Worksheet/Answer key Ecosystems, food chains and food webs
Ecosystems, food chains and food webs  Vocabulary/Answer key
Vocabulary/Answer key Ecosystems, food chains and food webs
Ecosystems, food chains and food webs  Vocabulary/Answer key
Vocabulary/Answer key Ecosystems, food chains and food webs
Ecosystems, food chains and food webs  Vocabulary/Answer key
Vocabulary/Answer key Ecosystems, food chains and food webs
Ecosystems, food chains and food webs  Vocabulary/Answer key
Vocabulary/Answer key Ecosystems, food chains and food webs
Ecosystems, food chains and food webs  Vocabulary/Answer key
Vocabulary/Answer key Ecosystems, food chains and food webs
Ecosystems, food chains and food webs 

 Activity Lesson
Activity Lesson
 Worksheet/Answer key
Worksheet/Answer key
 Worksheet/Answer key
Worksheet/Answer key
 Worksheet/Answer key
Worksheet/Answer key
 Vocabulary/Answer key
Vocabulary/Answer key
 Vocabulary/Answer key
Vocabulary/Answer key
 Vocabulary/Answer key
Vocabulary/Answer key
 Vocabulary/Answer key
Vocabulary/Answer key
 Vocabulary/Answer key
Vocabulary/Answer key

The resources above cover the following skills:
LIFE SCIENCE
Ecosystems: Interactions, Energy, and Dynamics
Examine the cycling of matter between abiotic and biotic parts of ecosystems to explain the flow of energy and the conservation of matter.
Analyze and interpret data to provide evidence regarding how resource availability impacts individual organisms as well as populations of organisms within an ecosystem.
Construct an explanation to predict patterns of interactions in different ecosystems in terms of the relationships between and among organisms (e.g., competition, predation, mutualism, commensalism, parasitism).
Engage in argument to defend the effectiveness of a design solution that maintains biodiversity and ecosystem services (e.g., using scientific, economic, and social considerations regarding purifying water, recycling nutrients, preventing soil erosion).