Formation of Anions
Anions are formed through the process of electron gain. Non-metallic elements tend to gain electrons to achieve a stable outer electron configuration, typically with eight electrons in their outermost energy level. This results in the formation of negatively charged ions.Naming Convention for Anions
Anions are named by adding the suffix "-ide" to the root of the element's name. For example, when chlorine gains an electron to form an anion, it is named chloride (Cl-).Properties of Anions
- Anions are larger in size compared to their parent atoms due to the addition of extra electrons, which increases the electron-electron repulsion. - Anions are attracted to the positive terminal of a battery or external electric field due to their negative charge. - Anions can form ionic compounds with cations (positively charged ions) through electrostatic interactions.Study Guide for Anions
To study anions effectively, consider the following key points:- Understand the process of electron gain and the formation of anions.
- Learn the naming convention for anions and practice naming common anions formed by non-metallic elements.
- Explore the properties of anions, including their size, charge, and behavior in electric fields.
- Practice writing chemical formulas for ionic compounds formed by anions and cations.
- Engage in hands-on activities or simulations to visualize the behavior of anions in different chemical scenarios.
◂Science Worksheets and Study Guides Seventh Grade. Our Solar System
Study Guide Our Solar System
Our Solar System  Activity Lesson
Activity Lesson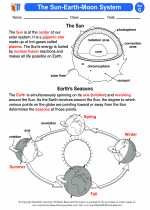 The Sun-Earth-Moon System
The Sun-Earth-Moon System  Activity Lesson
Activity Lesson Our Solar System
Our Solar System  Worksheet/Answer key
Worksheet/Answer key Our Solar System
Our Solar System  Worksheet/Answer key
Worksheet/Answer key Our Solar System
Our Solar System  Worksheet/Answer key
Worksheet/Answer key Our Solar System
Our Solar System  Worksheet/Answer key
Worksheet/Answer key Our Solar System
Our Solar System  Vocabulary/Answer key
Vocabulary/Answer key Our Solar System
Our Solar System  Vocabulary/Answer key
Vocabulary/Answer key Our Solar System
Our Solar System  Vocabulary/Answer key
Vocabulary/Answer key Our Solar System
Our Solar System 

 Activity Lesson
Activity Lesson
 Activity Lesson
Activity Lesson
 Worksheet/Answer key
Worksheet/Answer key
 Worksheet/Answer key
Worksheet/Answer key
 Worksheet/Answer key
Worksheet/Answer key
 Worksheet/Answer key
Worksheet/Answer key
 Vocabulary/Answer key
Vocabulary/Answer key
 Vocabulary/Answer key
Vocabulary/Answer key
 Vocabulary/Answer key
Vocabulary/Answer key
