Our Solar System -> boyle's law
Boyle's Law
Boyle's law is a fundamental principle in physics that describes the relationship between the pressure and volume of a gas at constant temperature. It states that the pressure of a given mass of gas is inversely proportional to its volume.
Mathematical Representation
The mathematical representation of Boyle's law is:
P1V1 = P2V2
Where P1 and V1 are the initial pressure and volume, and P2 and V2 are the final pressure and volume of the gas, respectively.
Explanation
Boyle's law can be understood through the behavior of gas molecules. When the volume of a gas is decreased, the gas molecules are forced to occupy a smaller space, leading to more frequent collisions with the walls of the container. This increase in collisions results in a higher pressure. Conversely, when the volume of a gas is increased, the gas molecules have more space to move and collide with the container walls less frequently, leading to a lower pressure.
Applications
Boyle's law has numerous practical applications, including in scuba diving, where it is used to calculate air consumption at different depths, and in the functioning of internal combustion engines, where it governs the compression and expansion of gases within the cylinders.
Study Guide
.◂Science Worksheets and Study Guides Seventh Grade. Our Solar System

 Activity Lesson
Activity Lesson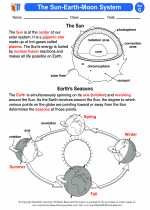
 Activity Lesson
Activity Lesson
 Worksheet/Answer key
Worksheet/Answer key
 Worksheet/Answer key
Worksheet/Answer key
 Worksheet/Answer key
Worksheet/Answer key
 Worksheet/Answer key
Worksheet/Answer key
 Vocabulary/Answer key
Vocabulary/Answer key
 Vocabulary/Answer key
Vocabulary/Answer key
 Vocabulary/Answer key
Vocabulary/Answer key
