Our Solar System -> charles's law
Charles's Law
Charles's Law states that the volume of a gas is directly proportional to its temperature, given that pressure and amount of gas remain constant. In other words, as the temperature of a gas increases, the volume of the gas also increases, and vice versa.
Mathematical Representation
The mathematical representation of Charles's Law is:
V1 / T1 = V2 / T2
Where: V1 = initial volume of the gas T1 = initial temperature of the gas (in Kelvin) V2 = final volume of the gas T2 = final temperature of the gas (in Kelvin)
Application and Practical Examples
Charles's Law is often observed in everyday situations. For example, when a balloon is heated, the air inside the balloon expands, causing the balloon to inflate. This is because the increase in temperature leads to an increase in the volume of the gas inside the balloon, in accordance with Charles's Law.
Study Guide
- Define Charles's Law.
- What are the conditions under which Charles's Law is applicable?
- State the mathematical representation of Charles's Law.
- Provide an example of a real-life application of Charles's Law.
- How is temperature measured in the context of Charles's Law?
- Explain the relationship between volume and temperature as per Charles's Law.
- Discuss the significance of using Kelvin as the unit of temperature in Charles's Law calculations.
By understanding and mastering Charles's Law, you will be able to predict the behavior of gases with changes in temperature and volume, and apply this knowledge to various scientific and practical scenarios.
.◂Science Worksheets and Study Guides Seventh Grade. Our Solar System

 Activity Lesson
Activity Lesson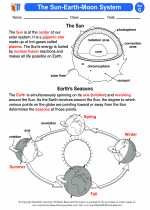
 Activity Lesson
Activity Lesson
 Worksheet/Answer key
Worksheet/Answer key
 Worksheet/Answer key
Worksheet/Answer key
 Worksheet/Answer key
Worksheet/Answer key
 Worksheet/Answer key
Worksheet/Answer key
 Vocabulary/Answer key
Vocabulary/Answer key
 Vocabulary/Answer key
Vocabulary/Answer key
 Vocabulary/Answer key
Vocabulary/Answer key
