Our Solar System -> periodic table
What is the Periodic Table?
The periodic table is a tabular arrangement of the chemical elements, organized by their atomic number, electron configuration, and recurring chemical properties. It provides a useful framework for analyzing and understanding the behavior of elements and their compounds.
Structure of the Periodic Table
The periodic table is organized into rows called periods and columns called groups. Elements in the same group share similar chemical properties, while elements in the same period have the same number of electron shells.
Key Concepts to Understand
- Atomic Number: The number of protons in the nucleus of an atom, which determines an element's identity in the periodic table.
- Atomic Symbol: The unique shorthand representation of each element, usually one or two letters.
- Groups: Vertical columns on the periodic table that contain elements with similar chemical properties.
- Periods: Horizontal rows on the periodic table that represent the number of electron shells in an element's atom.
Study Tips
When studying the periodic table, it's helpful to focus on the following:
- Memorize the first 20 elements and their symbols.
- Understand the trends in the periodic table, such as the increase in atomic number and the arrangement of elements within periods and groups.
- Practice identifying elements based on their position in the periodic table and predicting their chemical properties based on their group.
Practice Questions
- What is the atomic number of carbon?
- Which group contains the noble gases?
- How many periods are there in the periodic table?
[Periodic Table] Related Worksheets and Study Guides:
.◂Science Worksheets and Study Guides Seventh Grade. Our Solar System
Study Guide Our Solar System
Our Solar System  Activity Lesson
Activity Lesson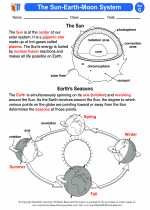 The Sun-Earth-Moon System
The Sun-Earth-Moon System  Activity Lesson
Activity Lesson Our Solar System
Our Solar System  Worksheet/Answer key
Worksheet/Answer key Our Solar System
Our Solar System  Worksheet/Answer key
Worksheet/Answer key Our Solar System
Our Solar System  Worksheet/Answer key
Worksheet/Answer key Our Solar System
Our Solar System  Worksheet/Answer key
Worksheet/Answer key Our Solar System
Our Solar System  Vocabulary/Answer key
Vocabulary/Answer key Our Solar System
Our Solar System  Vocabulary/Answer key
Vocabulary/Answer key Our Solar System
Our Solar System  Vocabulary/Answer key
Vocabulary/Answer key Our Solar System
Our Solar System 

 Activity Lesson
Activity Lesson
 Activity Lesson
Activity Lesson
 Worksheet/Answer key
Worksheet/Answer key
 Worksheet/Answer key
Worksheet/Answer key
 Worksheet/Answer key
Worksheet/Answer key
 Worksheet/Answer key
Worksheet/Answer key
 Vocabulary/Answer key
Vocabulary/Answer key
 Vocabulary/Answer key
Vocabulary/Answer key
 Vocabulary/Answer key
Vocabulary/Answer key
