Recrystallization
Recrystallization is a technique used to purify solid compounds. It involves dissolving the impure compound in a hot solvent, allowing it to recrystallize as the solution cools, and then isolating the pure crystals from the solution. This process is based on the differences in solubility of the desired compound and impurities in a given solvent.
Key Concepts
- Solubility: Understanding the solubility of the compound in different solvents is crucial for selecting the appropriate solvent for recrystallization.
- Impurities: The impurities present in the compound may have different solubilities than the desired compound, leading to their separation during recrystallization.
- Crystallization: The formation of pure crystals as the solution cools is a result of the decreased solubility of the compound at lower temperatures.
- Filtration: Isolating the pure crystals from the solution often involves filtration and subsequent washing of the crystals to remove any remaining impurities.
Study Guide
Here are some key points to focus on when studying recrystallization:
- Understand the concept of solubility and how it impacts the selection of a suitable solvent for recrystallization.
- Learn the techniques for assessing the purity of a compound before and after recrystallization.
- Explore the factors that can affect the success of a recrystallization process, such as temperature, rate of cooling, and choice of solvent.
- Practice solving problems related to calculating the yield and purity of recrystallized compounds.
- Review the steps involved in the recrystallization process, from dissolving the impure compound to isolating the pure crystals.
By mastering these key concepts and study guide points, you will develop a strong understanding of recrystallization and its importance in purifying solid compounds.
.◂Science Worksheets and Study Guides Seventh Grade. Our Solar System
Study Guide Our Solar System
Our Solar System  Activity Lesson
Activity Lesson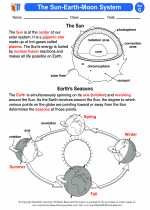 The Sun-Earth-Moon System
The Sun-Earth-Moon System  Activity Lesson
Activity Lesson Our Solar System
Our Solar System  Worksheet/Answer key
Worksheet/Answer key Our Solar System
Our Solar System  Worksheet/Answer key
Worksheet/Answer key Our Solar System
Our Solar System  Worksheet/Answer key
Worksheet/Answer key Our Solar System
Our Solar System  Worksheet/Answer key
Worksheet/Answer key Our Solar System
Our Solar System  Vocabulary/Answer key
Vocabulary/Answer key Our Solar System
Our Solar System  Vocabulary/Answer key
Vocabulary/Answer key Our Solar System
Our Solar System  Vocabulary/Answer key
Vocabulary/Answer key Our Solar System
Our Solar System 

 Activity Lesson
Activity Lesson
 Activity Lesson
Activity Lesson
 Worksheet/Answer key
Worksheet/Answer key
 Worksheet/Answer key
Worksheet/Answer key
 Worksheet/Answer key
Worksheet/Answer key
 Worksheet/Answer key
Worksheet/Answer key
 Vocabulary/Answer key
Vocabulary/Answer key
 Vocabulary/Answer key
Vocabulary/Answer key
 Vocabulary/Answer key
Vocabulary/Answer key
