Covalent Bonding
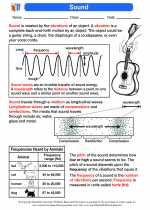
Covalent bonding is a type of chemical bond that involves the sharing of electron pairs between atoms. It occurs between non-metal atoms and is characterized by the sharing of valence electrons to achieve a stable electron configuration.
Key Concepts
- Electron Sharing: In a covalent bond, two atoms share one or more pairs of electrons to attain a stable configuration.
- Electronegativity: The ability of an atom to attract shared electrons. It determines the unequal sharing of electrons in a covalent bond.
- Types of Covalent Bonds: Single, double, and triple covalent bonds are formed by the sharing of one, two, or three pairs of electrons, respectively.
- Molecular Structure: Covalent bonding gives rise to various molecular shapes, including linear, trigonal planar, tetrahedral, and more, based on the number of shared electron pairs and lone pairs around the central atom.
- Polarity: Covalent bonds can be polar or nonpolar depending on the difference in electronegativity between the atoms involved.
Study Guide
- What is a covalent bond?
- How is a covalent bond formed?
- What is electronegativity and how does it influence covalent bonding?
- Explain the concept of electron sharing in covalent bonds.
- Describe the types of covalent bonds and provide examples of molecules with each type.
- How does covalent bonding contribute to the formation of molecular shapes?
- Differentiate between polar and nonpolar covalent bonds.
- Discuss the role of covalent bonding in the properties of substances.
- Illustrate the process of drawing Lewis dot structures for covalent molecules.
- Explain the significance of covalent bonding in the formation of organic compounds.