Sulfide Minerals
Sulfide minerals are a group of minerals that contain the element sulfur (S) as a major component. They are often found in association with metal ores and are an important source of many valuable metals, including copper, lead, zinc, silver, and gold. Here are some key points to help you understand sulfide minerals:
Chemical Composition
Sulfide minerals are composed of metal cations and sulfide anions. The most common sulfide mineral is iron sulfide, known as pyrite (FeS2). Other common sulfide minerals include galena (PbS), chalcopyrite (CuFeS2), sphalerite (ZnS), and cinnabar (HgS).
Formation
Sulfide minerals form through a variety of processes, including hydrothermal deposition, volcanic activity, and sedimentary processes. Hydrothermal fluids rich in sulfur can precipitate sulfide minerals as they cool and interact with rocks in the Earth's crust. Volcanic emissions can also lead to the formation of sulfide minerals in the surrounding environment.
Physical Properties
Sulfide minerals are generally dense and have metallic luster. They often form distinctive crystal shapes and can exhibit a range of colors, from metallic gray to brassy yellow and dark black. Many sulfide minerals are brittle and have a high specific gravity due to their metallic content.
Uses
Sulfide minerals are important sources of metals used in various industries. For example, galena is a major source of lead, sphalerite is the primary ore of zinc, and chalcopyrite is the main copper ore. These minerals are mined and processed to extract the valuable metals they contain, which are essential for manufacturing and technology.
Study Guide
.◂Science Worksheets and Study Guides Eighth Grade. The Endocrine system and Reproduction
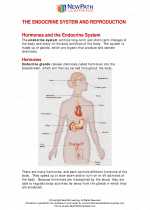
 Worksheet/Answer key
Worksheet/Answer key
 Worksheet/Answer key
Worksheet/Answer key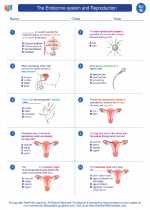
 Worksheet/Answer key
Worksheet/Answer key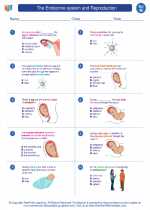
 Vocabulary/Answer key
Vocabulary/Answer key
 Vocabulary/Answer key
Vocabulary/Answer key
 Vocabulary/Answer key
Vocabulary/Answer key
 Vocabulary/Answer key
Vocabulary/Answer key
 Vocabulary/Answer key
Vocabulary/Answer key
