Uranium: An Overview
Uranium is a chemical element with the symbol U and atomic number 92. It is a silvery-grey metal in the actinide series of the periodic table. Uranium has the highest atomic weight of the primordially occurring elements. It occurs naturally in low concentrations of a few parts per million in soil, rock, and water, and is commercially extracted from uranium-bearing minerals such as uraninite.
Key Facts about Uranium
- Symbol: U
- Atomic number: 92
- Atomic weight: 238.02891 u
- State at room temperature: Solid
- Uses: Nuclear fuel for power plants, military applications, medical isotopes
Study Guide: Understanding Uranium
When studying uranium, it's important to understand its properties, uses, and its role in nuclear reactions. Here are some key topics to focus on:
1. Atomic Structure
Study the atomic structure of uranium, including the number of protons, neutrons, and electrons. Understand its position in the periodic table and its relationship to other elements.
2. Isotopes
Explore the different isotopes of uranium, with a focus on uranium-235 and uranium-238. Understand their stability, radioactive decay, and significance in nuclear reactions.
3. Nuclear Reactions
Learn about the role of uranium in nuclear fission reactions, including the process of nuclear energy generation in power plants and the production of nuclear weapons.
4. Environmental and Health Impacts
Examine the environmental and health impacts of uranium mining, processing, and waste disposal. Understand the potential hazards associated with exposure to uranium and its decay products.
5. Future Applications
Explore the potential future applications of uranium, including advanced nuclear reactor designs, nuclear propulsion systems, and the development of new medical isotopes for diagnostic and therapeutic purposes.
.◂Science Worksheets and Study Guides Eighth Grade. The Endocrine system and Reproduction
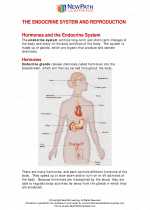
 Worksheet/Answer key
Worksheet/Answer key
 Worksheet/Answer key
Worksheet/Answer key
 Worksheet/Answer key
Worksheet/Answer key
 Vocabulary/Answer key
Vocabulary/Answer key
 Vocabulary/Answer key
Vocabulary/Answer key
 Vocabulary/Answer key
Vocabulary/Answer key
 Vocabulary/Answer key
Vocabulary/Answer key
 Vocabulary/Answer key
Vocabulary/Answer key
