What is Surface Tension?
Surface tension is the property of a liquid that allows it to resist external force. It is a result of the cohesive forces between the molecules in the liquid. These cohesive forces cause the molecules at the surface of the liquid to behave as if they are under tension, creating a "skin" on the surface of the liquid. This is what allows certain objects to float on the surface of water and enables water droplets to form spherical shapes.
Understanding Surface Tension
To understand surface tension better, let's consider the example of water striders, insects that can walk on the surface of water without sinking. This is possible due to the surface tension of water, which creates a thin film that can support the weight of the insects. Similarly, when you overfill a glass of water, the water forms a convex meniscus above the rim of the glass due to surface tension.
Factors Affecting Surface Tension
Surface tension is influenced by several factors, including the type of liquid, temperature, and the presence of impurities. For example, water has a higher surface tension than many other liquids, while adding soap to water can reduce its surface tension.
Experiments to Explore Surface Tension
There are several simple experiments that can help demonstrate surface tension. One common experiment involves placing a paper clip on the surface of water, where it floats due to the surface tension. Another experiment involves observing how water forms droplets on a smooth surface, showcasing the cohesive forces and surface tension of water molecules.
Study Guide for Surface Tension
As you study surface tension, consider the following key points:
- Define surface tension and explain its underlying principles.
- Explore real-life examples of surface tension, such as insect behavior on water surfaces.
- Investigate the factors that can affect surface tension, including temperature and impurities.
- Perform simple experiments to observe and measure surface tension in action.
- Consider the implications of surface tension in various applications, such as in the formation of droplets and bubbles.
By understanding the concept of surface tension and its applications, you can gain a deeper appreciation for the behavior of liquids and the forces that govern their interactions.
.◂Science Worksheets and Study Guides Fourth Grade. Organ systems

 Worksheet/Answer key
Worksheet/Answer key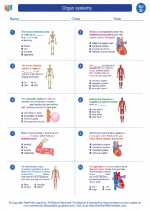
 Worksheet/Answer key
Worksheet/Answer key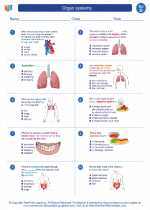
 Worksheet/Answer key
Worksheet/Answer key
 Vocabulary/Answer key
Vocabulary/Answer key
 Vocabulary/Answer key
Vocabulary/Answer key
 Vocabulary/Answer key
Vocabulary/Answer key
 Vocabulary/Answer key
Vocabulary/Answer key
 Vocabulary/Answer key
Vocabulary/Answer key
