Liquids: Properties and Characteristics
Liquids are one of the three states of matter, along with solids and gases. They have distinct properties and characteristics that set them apart from the other states of matter.
Properties of Liquids:
- Fluidity: Liquids can flow and take the shape of their container, making them fluid in nature.
- Volume: Liquids have a definite volume, but they do not have a definite shape.
- Density: Liquids have a relatively high density compared to gases but lower density compared to solids.
- Compressibility: Liquids are not easily compressible compared to gases, but they can still be compressed to some extent.
- Surface Tension: Liquids have surface tension, which allows them to form droplets and exhibit cohesive and adhesive properties.
- Viscosity: Liquids have varying viscosities, which determine their resistance to flow.
Characteristics of Liquids:
- Boiling Point and Freezing Point: Liquids have specific temperatures at which they boil and freeze, known as the boiling point and freezing point, respectively.
- Expansion: Like most substances, liquids expand when heated and contract when cooled.
- Capillarity: Liquids can be drawn into narrow spaces or tubes through capillary action, which is the result of adhesive and cohesive forces.
- Diffusion: Liquids can undergo diffusion, where their particles spread out to fill the available space.
- Evaporation and Condensation: Liquids can evaporate into gases when heated and condense back into liquids when cooled.
Study Guide:
When studying liquids, it's important to understand their properties and characteristics, as well as the factors that influence their behavior. Here are some key points to focus on:
- Learn about the molecular structure of liquids and how it differs from solids and gases.
- Understand the concept of density and how it relates to the mass and volume of liquids.
- Explore the effects of temperature and pressure on the behavior of liquids, including changes in state (boiling and freezing).
- Investigate the role of surface tension and viscosity in determining the flow and behavior of liquids.
- Examine real-life examples of liquids and their applications in various industries, such as in the form of solvents, lubricants, and cooling agents.
By grasping these fundamental concepts and delving into the properties and behavior of liquids, you can develop a comprehensive understanding of this essential state of matter.
[Liquid] Related Worksheets and Study Guides:
.◂Science Worksheets and Study Guides Fifth Grade. Flowers and seeds
Study Guide Flowers and seeds
Flowers and seeds  Activity Lesson
Activity Lesson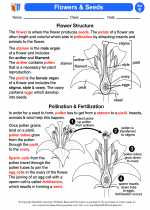 Flowers & Seeds
Flowers & Seeds  Worksheet/Answer key
Worksheet/Answer key Flowers and seeds
Flowers and seeds  Worksheet/Answer key
Worksheet/Answer key Flowers and seeds
Flowers and seeds  Worksheet/Answer key
Worksheet/Answer key Flowers and seeds
Flowers and seeds  Worksheet/Answer key
Worksheet/Answer key Flowers and seeds
Flowers and seeds  Vocabulary/Answer key
Vocabulary/Answer key Flowers and seeds
Flowers and seeds  Vocabulary/Answer key
Vocabulary/Answer key Flowers and seeds
Flowers and seeds 

 Activity Lesson
Activity Lesson
 Worksheet/Answer key
Worksheet/Answer key
 Worksheet/Answer key
Worksheet/Answer key
 Worksheet/Answer key
Worksheet/Answer key
 Worksheet/Answer key
Worksheet/Answer key
 Vocabulary/Answer key
Vocabulary/Answer key
 Vocabulary/Answer key
Vocabulary/Answer key

The resources above cover the following skills:
Life Science
All organisms have structures and systems with separate functions. Students can:
Develop and communicate an evidence-based scientific explanation of the role of different organs or structures that are important for an organism's survival - in both plants and animals