Protons
Protons are subatomic particles that are found in the nucleus of an atom. They have a positive electric charge and are essential for the structure and stability of the atom. The number of protons in the nucleus determines the identity of the atom and is known as the atomic number. For example, all atoms with 6 protons are carbon atoms, while atoms with 47 protons are silver atoms.
Key Facts About Protons:
- Protons are positively charged subatomic particles.
- They are found in the nucleus of an atom.
- The number of protons determines the identity of the atom.
- Protons are essential for the stability of the atom.
Study Guide for Protons:
- What is the charge of a proton?
- Where are protons found in an atom?
- What is the significance of the number of protons in an atom?
- What is the relationship between protons and the stability of an atom?
The charge of a proton is positive (+1).
Protons are found in the nucleus of an atom.
The number of protons determines the identity of the atom and is known as the atomic number.
Protons are essential for the stability of the atom. The balance between the positively charged protons and the negatively charged electrons determines the overall stability of the atom.
[Protons] Related Worksheets and Study Guides:
.◂Science Worksheets and Study Guides Seventh Grade. Our Solar System
Study Guide Our Solar System
Our Solar System  Activity Lesson
Activity Lesson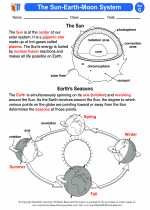 The Sun-Earth-Moon System
The Sun-Earth-Moon System  Activity Lesson
Activity Lesson Our Solar System
Our Solar System  Worksheet/Answer key
Worksheet/Answer key Our Solar System
Our Solar System  Worksheet/Answer key
Worksheet/Answer key Our Solar System
Our Solar System  Worksheet/Answer key
Worksheet/Answer key Our Solar System
Our Solar System  Worksheet/Answer key
Worksheet/Answer key Our Solar System
Our Solar System  Vocabulary/Answer key
Vocabulary/Answer key Our Solar System
Our Solar System  Vocabulary/Answer key
Vocabulary/Answer key Our Solar System
Our Solar System  Vocabulary/Answer key
Vocabulary/Answer key Our Solar System
Our Solar System 

 Activity Lesson
Activity Lesson
 Activity Lesson
Activity Lesson
 Worksheet/Answer key
Worksheet/Answer key
 Worksheet/Answer key
Worksheet/Answer key
 Worksheet/Answer key
Worksheet/Answer key
 Worksheet/Answer key
Worksheet/Answer key
 Vocabulary/Answer key
Vocabulary/Answer key
 Vocabulary/Answer key
Vocabulary/Answer key
 Vocabulary/Answer key
Vocabulary/Answer key
